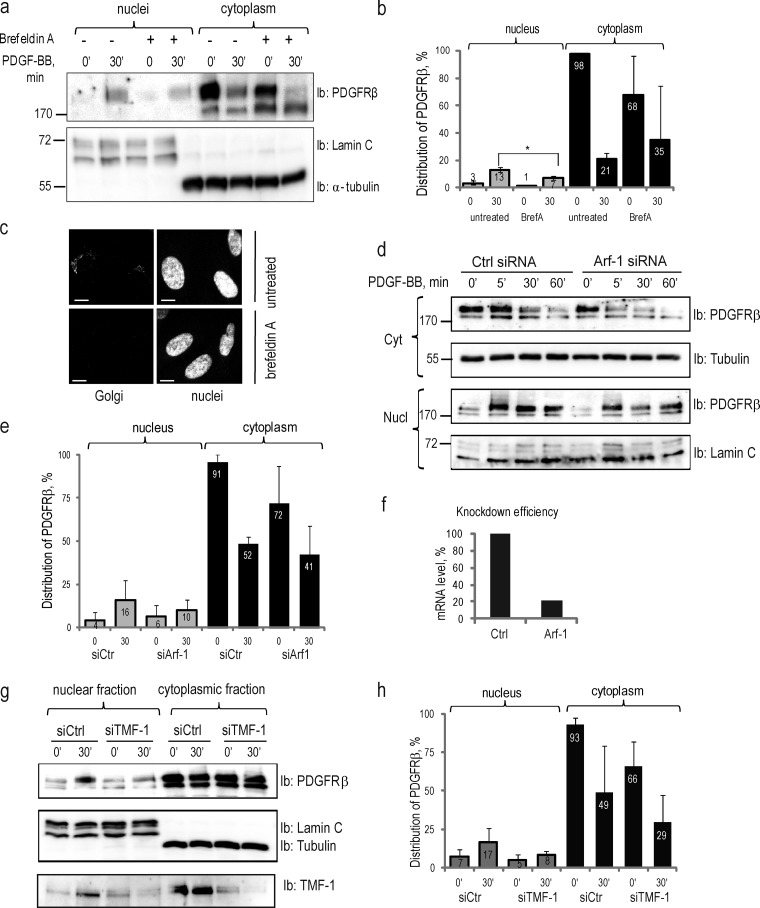
Figure 4.
Nuclear translocation of PDGFRβ is dependent on intact Golgi and retrograde transport system. (a) Inhibition of nuclear translocation of PDGFRβ upon brefeldin A treatment. Nuclear and cytoplasmic fractions of cells treated or not with brefeldin A were immunoblotted for PDGFRβ (top) and markers (bottom). (b) Quantification of the distribution of PDGFR in the nucleus and the cytoplasm upon treatment with brefeldin A was performed, as described above, for three independent experiments. *, P < 0.05, two-tailed t test. (c) The integrity of the Golgi apparatus was lost upon treatment with brefeldin A. Golgi (top left) was absent after the treatment with brefeldin A (bottom left); nuclei are presented on images to the right. Bars, 10 µm. (d) PDGFRβ translocation to the nucleus is decreased upon siRNA knockdown of Arf-1. Cytoplasmic (two top panels) and nuclear (two bottom panels) fractions were immunoblotted for PDGFRβ; the purity of the fractions was determined by immunoblotting for α-tubulin and lamin C. (e) Nuclear distribution of PDGFRβ upon Arf-1 knockdown was quantified based on three independent experiments. (f) The efficiency of Arf-1 depletion was confirmed by quantitative PCR. The mean mRNA level in knockdown samples was calculated as a percentage relative to the mean mRNA levels in control samples. (g) Nuclear translocation of PDGFRβ is decreased upon siRNA knockdown of TMF-1. Nuclear and cytoplasmic fractions were immunoblotted with PDGFRβ antibody (top) or lamin A/C and α-tubulin (middle). The efficiency of TMF-1 knockdown was demonstrated by immunoblotting with TMF-1 antibody (bottom). (h) The distribution of PDGFRβ in the nucleus and cytoplasm upon depletion of TMF-1 was quantified as described above based on three independent experiments. Molecular mass was measured in kilodaltons. Error bars indicate SD. Ib, immunoblotting.