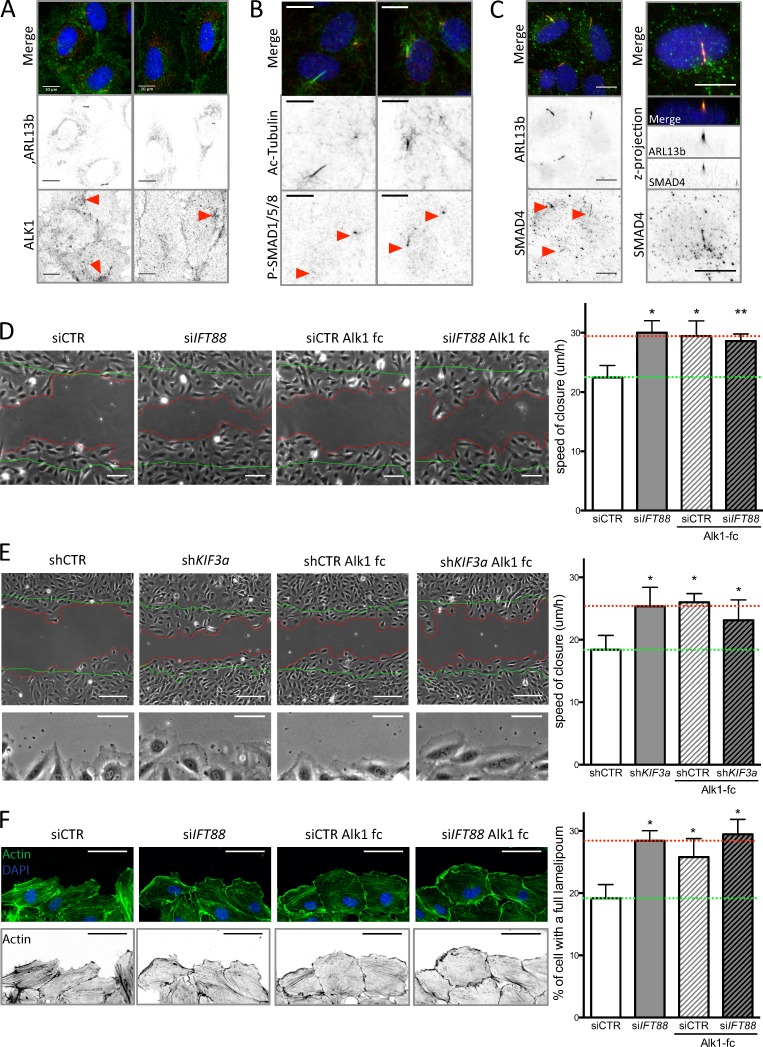
Figure 5.
Loss of BMP9 signaling through the cilium increases EC migration. (A) HUVECs were stained for ARL13b (primary cilium; red), ALK1 (green), and DAPI (blue; methanol fixation, n = 2) under static conditions. Bars, 10 µm. (B) HUVECs were stained for acetylated tubulin (primary cilium; green), P-SMAD1/5/8 (red), and DAPI (blue; methanol fixation, n = 2) under LSS conditions. Bars, 10 µm. (C) HUVECs were stained for ARL13b (primary cilium; red), SMAD4 (green), and DAPI (blue; methanol fixation, n = 2) under LSS conditions. Bars, 10 µm. (D) A wound-closure assay was performed on ECs transfected with siCTR and siIFT88, treated or not with a BMP9 trap, an ALK1-Fc receptorbody (25 ng/ml, for the duration of the wound experiment). Representative images were taken 9 h after the wound was made (green lines, wound at t = 0; red lines, wound at t = 9 h) and the speed of closure was quantified (n = 5; mean ± SEM; two-sided Wilcoxon test; *, P < 0.05; **, P < 0.01). Bars, 100 µm. (E) A wound-closure assay was performed on ECs transfected with shCTR and shKIF3a, treated or not with ALK1-Fc (25 ng/ml, for the duration of the wound experiment). Representative images were taken 9 h after the wound was made (green lines, wound at t = 0; red lines, wound at t = 9 h) and the speed of closure was quantified (n = 7; mean ± SEM; two-sided Wilcoxon test; *, P < 0.05; **, P < 0.01). Bars, 150 µm. Bottom images of the panel highlight lamellipodia formation in shKIF3a, ALK1-fc, and shKIF3a+ALK1-fc condition compared with control. Bars, 60 µm. (F) Representative images of actin staining (phalloidin–Alexa Fluor 594; green, top panel; black, bottom panel) of the ECs leading the wound closure and quantification of the number of cell with an undisrupted front lamellipodium (observed after 6 h of wound; n = 3; two-sided paired t test. Data distribution was assumed to be normal, but this was not formally tested. *, P < 0.05. Bars, 60 µm.