Figure 1. Existing antiviral targets and therapies.
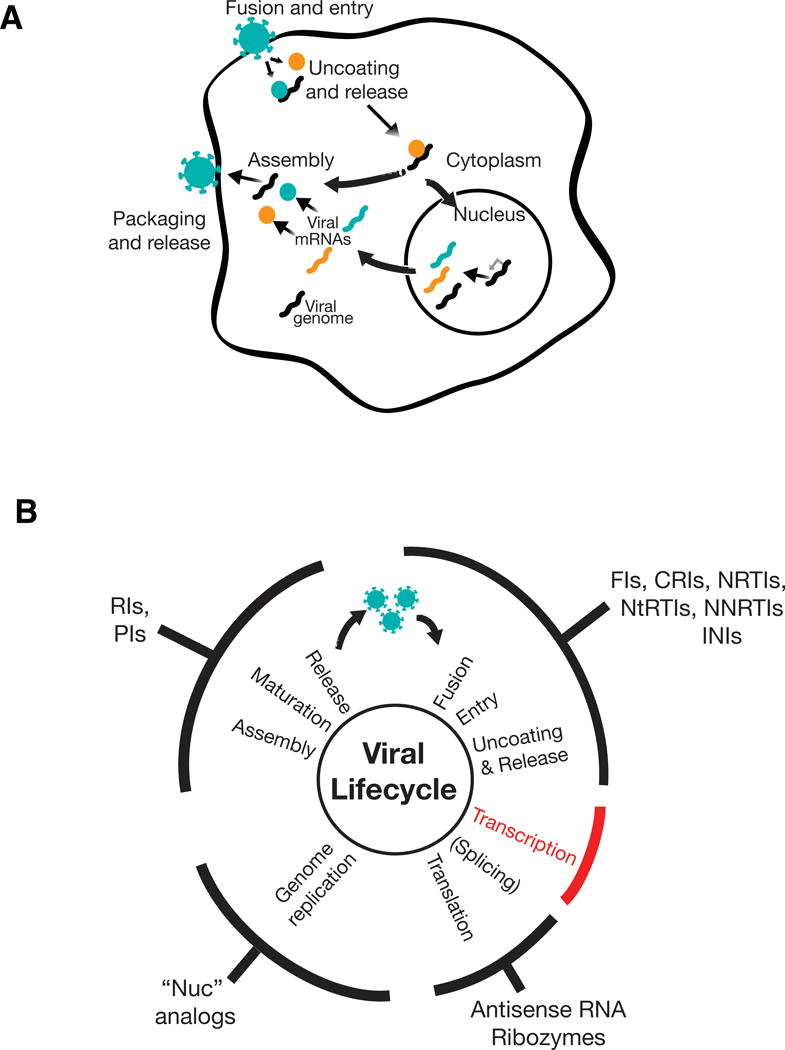
A) General schematic of the viral lifecycle. (i) Viruses recognize specific receptors and associated components on the surface of their target host cells and fuse with the host cell membrane. (ii) This fusion leads to viral entry into the cytoplasm followed by uncoating of the virus and release of components that include the viral proteins (colored circles) and viral RNA or DNA (black squiggle). (iii) The released viral components either induce viral transcription in the nucleus (e.g., DNA viruses other than poxviruses and retroviruses; mRNAs shown in colored squiggles) or directly undergo genome replication in the cytoplasm (e.g., RNA viruses and poxviruses). Genome replication can also be considered to be coincident with transcription (e.g., Influenza or retroviruses, where the genomic RNA is a full-length unspliced mRNA transcript) or follow translation aided by virus-generated products (e.g., Poliovirus). (iv) Viral genomes and products assemble, are packaged into virions, and released from the cell.
B) Targeting stages of the viral lifecyle for antiviral therapies. The generalized viral life cycle (discussed in panel A) is represented by the inner circle with classes of approved antivirals that affect these stages noted on the outer circumference. Antivirals against transcription are notably absent (red). Antivirals abbreviations are as follows (together with representative FDA-approved example): FI—fusion inhibitor (e.g., Enfuvirtide for HIV); CRI—co-receptor inhibitor (e.g., Maraviroc for HIV); NNRTI—non-nucleoside reverse transcriptase inhibitor (e.g., Efavirenz for HIV), NRTI—nucleoside reverse transcriptase inhibitor (e.g., Azidothymidine, AZT, for HIV); NtRTI—nucleotide reverse transcriptase inhibitor (e.g., Tenofivir for HIV); INI—integrase inhibitor (e.g., Raltegravir for HIV); RI—release inhibitor (e.g., Zanamivir for Influenza), PI—protease inhibitor (e.g., Darunavir for HIV or Boceprevir for HCV), RI—release/uncoating inhibitor (e.g., Amantidine for Influenza). Approved RNA-based therapeutics include fomivirsen (antisense RNA) to treat CMV; ribozyme therapies remain experimental. A range of nucleic acid (“nuc”) analogs are FDA-approved to inhibit viral genome replication including Aciclovir for Herpes Simplex 1 (HSV-1), Ganciclovir for CMV, and Sofosbuvir for Hepatitis C Virus (HCV).
