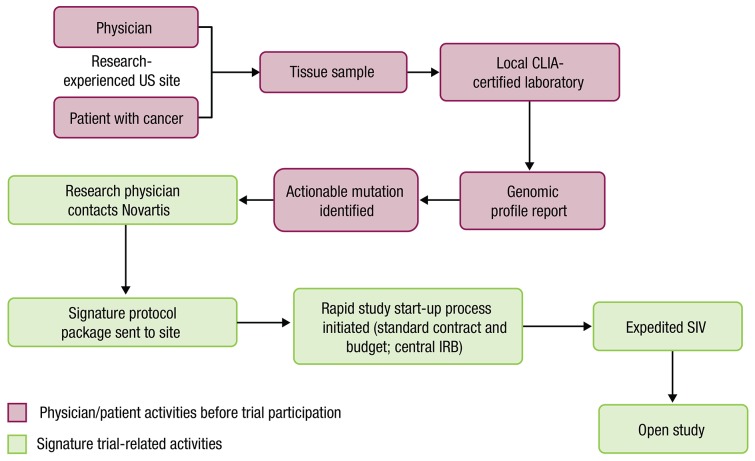
Figure 1. Signature Program protocol start-up process.
Each protocol excluded patients with certain tumor types, including those for which the agent being studied has shown no benefit and those for which key studies are planned or ongoing. CLIA, Clinical Laboratory Improvement Amendments; IRB, institutional review board; SIV, site initiation visit.