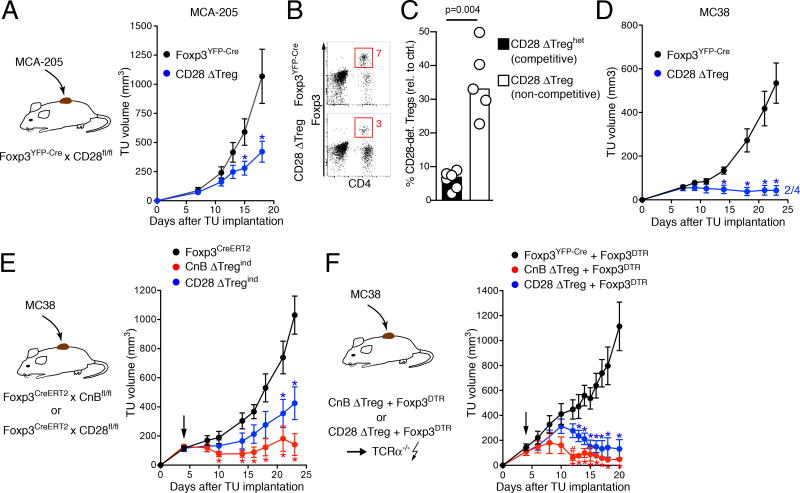
Figure 7. CnB-deficient Treg are more strongly impaired in maintaining tumor tolerance than CD28-deficient Treg.
(A) Growth rates of subcutaneous MCA-205 tumors CD28 ΔTreg (n=3) or Foxp3YFP-Cre control mice (n=3) . Means and SEM for one experiment of two are shown. (B) Frequency of Foxp3+ Treg in tumor tissue of Foxp3YFP-Cre and CD28 ΔTreg mice at day 17 or 18 after tumor implantation. Data are representative of 7 (Foxp3YFP-Cre) and 5 mice (CD28 ΔTreg). (C) Accumulation of CD28-deficient YFP+ Treg in tumor tissue is more strongly impaired in competitive (CD28 ΔTreghet hosts, n=6) than in non-competitive settings (CD28 ΔTreg hosts, n=5). CD28-deficient YFP+ Treg were compared with YFP+ Treg in Foxp3YFP-Cre/wt and Foxp3YFP-Cre hosts, respectively. Bars show means, p-value calculated by Mann-Whitney U test. (D) Growth of MC38 tumors in CD28 ΔTreg (n=4) mice or Foxp3YFP-Cre control mice (n=6) . Mean and SEM for are shown. (E) Growth of MC38 tumors in mice with inducible Treg-specific deletion of CnB or CD28. Tumor cells were implanted in Foxp3CreERT2, CnB ΔTregind, and CD28 ΔTregind mice. Arrow indicates start of tamoxifen administration, resulting in the deletion of CnB or CD28. Mean ± SEM and one experiment of two is shown, n=4 per group. (F) Growth of MC38 tumors in mixed chimeras generated in irradiated TCRα–/– hosts with Foxp3DTR and either CnB ΔTreg, CD28 ΔTreg or Foxp3YFP-Cre bone marrow. Upon administration of diphtheria toxin (arrow), mice are acutely converted into functional CnB ΔTreg or CD28 ΔTreg mice without pre-existing lymphoproliferative disease. Foxp3YFP-Cre + Foxp3DTR: n=5; CnB ΔTreg + Foxp3DTR: n=4; CD28 ΔTreg + Foxp3DTR: n=7. Mean ± SEM is shown.
In A, D, E, and F, * indicates p-values<0.05 against control mice, # indicates p-values<0.05 against CD28 ΔTreg mice, p-values calculated using muliple t-tests with Sidak post-test.