Abstract
Intricate processes in the thymus and periphery help curb the development and activation of autoreactive T cells. The subtle signals that govern these processes are an area of great interest, but tuning TCR sensitivity for the purpose of affecting T cell behavior remains technically challenging. Previously, our lab described the derivation of two TCR-transgenic CD4 T cell mouse lines, LLO56 and LLO118, which recognize the same cognate Listeria epitope with the same affinity. Despite the similarity of the two TCRs, LLO56 cells respond poorly in a primary infection while LLO118 cells respond robustly. Phenotypic examination of both lines revealed a substantial difference in their surface of expression of CD5, which serves as a dependable readout of the self-reactivity of a cell. We hypothesized that the increased interaction with self by the CD5-high LLO56 was mediated through TCR signaling, and was involved in the characteristic weak primary response of LLO56 to infection. To explore this issue, we generated an inducible knockin mouse expressing the self-sensitizing voltage-gated sodium channel Scn5a. Overexpression of Scn5a in peripheral T cells via the CD4-Cre promoter resulted in increased TCR-proximal signaling. Further, Scn5a-expressing LLO118 cells, after transfer into BL6 recipient mice, displayed an impaired response during infection relative to “wild-type” LLO118 cells. In this way, we were able to demonstrate that tuning of TCR sensitivity to self can be used to alter in vivo immune responses. Overall, these studies highlight the critical relationship between TCR:self-pMHC interaction and an immune response to infection.
Introduction
Every mature peripheral T cell begins its life by undergoing a finely tuned process of selection in the thymus, where its rearranged T cell receptor (TCR) interacts with self-peptide(s) displayed by thymic antigen presenting cells (APCs). This process begins with positive selection, during which the cell requires a minimum level of interaction with self to avoid the fate of death by neglect. During the process of positive selection, thymocytes are highly sensitized to developmental signaling cues (1). Synchronized expression of certain ion channels during positive selection is also key to T cell development. Our laboratory has previously demonstrated that the Scn5a/Scn4b voltage-gated Na+ channel (VGSC), which enables the sustained entry of Ca2+ into CD4+CD8+ double-positive (DP) thymocytes, is required for positive selection of CD4+ T cells in the thymus (2). In fact, ectopic expression of the human Scn5a/Scn4b voltage-gated sodium channel (VGSC) in CD4+ T cell hybridomas increased the sensitivity of the T cells to the extent that they were able to respond to their positively selecting ligand (2, 3). Scn5a, which forms the actual pore of the VGSC, is sufficient to enhance this ligand sensitivity in the absence of Scn4b, which serves as a modifier of the electrophysiological properties of the channel. After the CD4+CD8+ double-positive (DP) stage of thymocyte development, Scn5a expression is not detectable in T cells; it has been proposed that this prevents the autoreactivity of peripheral T cells (2).
Following positive selection is the process of negative selection. During this process, the body eliminates T cells that react too strongly with self-peptide:MHC, favoring cells that are relatively less reactive (4). Even after the immune system rids itself of highly self-reactive cells, it is still left with T cells representing a spectrum of responses to self-peptide:MHC. Some will be relatively more self-reactive than others, but will still be released as mature T cells into the periphery. Many of these, on the highest end of the truncated self-reactivity spectrum, are destined to become regulatory T cells (Tregs) (5–10). However, some of these newly generated T cells remain potential effector cells. How, then, can the immune system ensure these more self-reactive cells don’t become pathogenic, i.e., create unintended damage during the course of an infection/insult, or lead to the development of autoimmunity? The subtle signals that govern these protective mechanisms remain an area of great interest in T cell and autoimmunity research (9).
Once mature T cells exit the thymus and reach the periphery, tonic signaling is critical for their maintenance and homeostasis (11). Tonic signaling consists of low-level interactions between the TCR and self-peptide:MHC, and for CD4+ T cells requires peripheral expression of MHC class II (12). These interactions do not initiate full-fledged TCR signaling cascades and T cell activation; however, tonic signaling can subtly impact the activation state of the T cell (13, 14) and regulate gene expression levels (15, 16).
Expression levels of the glycoprotein CD5 (and other molecules, such as the orphan hormone receptor Nur77) are useful readouts for the TCR affinity for self, as maintained in the periphery via tonic signaling (17). It has been established that the greater the strength of T cell receptor (TCR) signal perceived during thymic development in response to self-peptide:MHC, the higher the resulting CD5 levels on peripheral circulating T cells (18). CD5 is an immunomodulatory surface molecule that is a member of the Scavenger-Receptor Cysteine-Rich (SRCR) superfamily, and clusters at the immune synapse upon TCR stimulation (19–24). Its expression on developing thymocytes is carefully regulated, and it can be found on the surface of mature CD8+ and CD4+ T cells, B-1 B cells, and select populations of dendritic cells (DCs) (18, 25, 26). The intracellular domain of CD5, which is absolutely required for its inhibitory function, contains four potential tyrosine phosphorylation sites, including an immunoreceptor tyrosine-based inhibition motif (ITIM) (27–29).
We have previously described the derivation and characterization of two Listeria-specific CD4+ T cell transgenic mouse lines, LLO56 and LLO118 (3, 30, 31). These two T cells recognize the same epitope of listeriolysin O (LLO190-205) with the same affinity. However, despite their in vitro similarity, LLO56 and LLO118 behave very differently in vivo. When 3000 of each cell is co-transferred into recipient B6 mice, many more LLO118 cells can be recovered from recipient mice at day 7 following infection. The LLO56 cells do respond in the primary response, but have increased cell death, resulting in the observed lower number of T cells at day 7. The LLO56 T cells conversely mount a very robust recall response upon reinfection. These differences led us to probe what characteristics of LLO56 and LLO118 led to their divergent responses. While these cells have overall very similar phenotypes in their naive state, they do differ greatly in their surface expression of CD5. Circulating LLO56 T cells express very high levels of CD5, indicating a higher degree of self-reactivity is inherent in their maintenance. We have also shown that LLO56 thymii have a greater number and frequency of CD4+ single-positive (SP) thymocytes, and a greater number of TCRhiCD69+ post-selection thymocytes (3). Collectively, these data indicate that, during development, LLO56 T cells perceive relatively stronger signals via their TCR than their LLO118 counterparts, and that these differences persist in the periphery are maintained via tonic signaling.
Changing the self-pMHC landscape to affect tonic signaling is technically challenging with current techniques. Therefore, we undertook the approach of changing the signaling threshold of the T cell, in order to change tonic signaling. As reported herein, we created a unique mouse with inducible expression of the VGSC Scn5a component. Using this system, we were able to modulate the CD4+ TCR signaling sensitivity, allowing us to directly test functional links between a T cell’s sensation of self and its response to infection. We did so without great perturbation to the immune system, as these mice do not differ from their wild-type littermates in thymocyte development, nor in T cell maturation. While CD4+ T cells from Scn5a-expressing mice are endowed with more sensitive and robust TCR-proximal signaling, they exhibit an unexpectedly impaired response to infection. We propose that this dampened response reflects a balance of positive and negative regulation of T cells in the periphery, necessary to prevent unwanted autoimmunity.
Materials and Methods
Mice
The LLO56 and LLO118 TCR transgenic lines, specific for listeriolysin O (190–205) (LLO190-205/I-Ab), have been previously described by our lab (3, 30). These mice are maintained on a Rag1-knockout background with homozygous congenic marker expression (LLO118-Ly5.1; LLO56-Thy1.1). CD4-Cre and Ert-Cre mice were obtained from The Jackson Laboratory (Bar Harbor, ME).
To create the Scn5a mice, a flox-stop-flox cassette from pCALSL-mir30 (a gift of Connie Cepko, Addgene plasmid #13786 (32)) was inserted upstream of the human Scn5a-GFP cDNA (Genecopoeia, NM_198056.2). This construct was targeted to the Rosa26 locus by electroporating into JM8.N4 C57BL/6N-derived ES cells (UC Davis KOMP Repository, (33)). Two successfully targeted ES clones were injected into blastocysts and both lines were transmitted in the germline. One line (#53) was selected to be bred to the Cre expressing strains.
All mice were bred and housed in specific pathogen free (SPF) conditions of the animal facility at Washington University Medical Center. All use of laboratory animals was approved and done in accordance with the Washington University Division of Comparative Medicine guidelines.
For Ert2-Cre induction, Tamoxifen (Sigma) was suspended in corn oil (Sigma) at a concentration of 100mg/mL. Mice were orally gavaged with 50µL (5mg) of the Tamoxifen solution, and then cardiac tissue was evaluated for GFP expression 24 hours later.
Bacterial Infections
The Listeria monocytogenes strain 1043S used in this study was generously provided by D. Portnoy (University of California, Berkeley, CA).
Flow cytometry
All samples were analyzed on BD FACSCanto II or BD LSRFortessa cytometers, and data were analyzed using FlowJo software (FlowJo, LLC). The following antibodies/clones were used for cell analysis: CD3ε (clone 145-2C11, FITC, BioLegend; clone 145-2C11, APC, BioLegend), CD4 (clone RM4.5, FITC, BioLegend; clone RM4.5, eFluor 450, eBioscience; clone RM4.5, PerCP-Cy5.5, eBioscience), CD5 (clone 53-7.3, FITC, BD Biosciences), CD8α (clone 53-6.7, APC, BD Biosciences), CD24 (clone MI/69, FITC, BioLegend), CD25 (clone PC61, PE, Biolegend), CD44 (clone IM7, FITC, BioLegend), CD45.1/Ly5.1 (clone A20, eFluor 450, eBioscience), CD62L (clone MEL-14, PE, BioLegend), CD69 (clone H1.2F3, PE-Cy7, BioLegend), CD90.1/Thy1.1 (clone OX-7, PE, BioLegend), CTLA-4 (clone UC10-4B9, APC, eBioscience), Foxp3 (clone FJK-16S, APC, eBioscience), PD-1 (clone RMPI-30, PE-Cy7, BioLegend), TCRβ (clone H57-597, PerCP-Cy5.5, BioLegend; clone H57-597, FITC, BD Biosciences).
For measurement of apoptosis, the PE Annexin V Apoptosis Kit I (BD Pharmingen) was used according to manufacturer’s instructions.
Intracellular Cytokine Staining
The production of IL-2 and IFNg was assessed after incubating splenocytes for 30 minutes with 1ng/ml PMA (Sigma-Aldrich) plus 1µg/ml ionomycin (Sigma-Aldrich), followed by incubation for an additional 4 hours in the presence of 2µg/ml brefeldin A (Sigma-Aldrich). For staining, the Foxp3/Transcription Factor Staining Buffer Kit (eBioscience) was used according to manufacturer’s instructions, along with antibodies against IL-2 (clone JES6-5H4, PE, BioLegend) and IFNγ (clone XMG1.2, APC, BioLegend).
p-PLCγ Measurement
Red blood cell lysis was performed on naïve splenocytes, which were then negatively bead enriched for CD4+ T cells (Mouse CD4+ T cell Kit, Miltenyi Biotec). For each time point, pre-complexed CD3e-IgG (CD3ε, clone 2C11, Biolegend; Biotin Goat anti-hamster, Biolegend) was added to 1.5×106 CD4+ T cells. Stimulation conditions were: unstimulated, 30s, 1 min., 3 min., 5 min., 10 min., and 15 min. Cells were then washed and lysed, and lysate supernatant was boiled for 5 min. before being loaded onto an 8% SDS-PAGE gel and run at 40 mAmps for 1 hour. The gel was then transferred to nitrocellulose for 16 hrs. at 15V. The membrane was then blocked in a 1:1 mixture of PBS and blocking buffer (Li-Cor) for 1 hour. After blocking, the blocking solution was removed and pPCLγ (Cell Signaling) and mouse b-actin (Biolegend) were each added to the nitrocellulose at 1:1000, in 1:1 PBS/Licor blocking buffer with 0.1% Tween-20. After a 2 hr. incubation, the nitrocellulose was washed. IR Dye 800CW (Li-Cor) and Alexa Flour 680 goat anti-rabbit IgG (Life Technologies) were each added at 1:12,500 and incubated for 1 hr. The membrane was again washed and then imaged on Li-Cor with a 700 intensity of 8 and an 800 intensity of 5.
Ca2+ Flux
Red blood cell lysis was performed on naïve splenocytes, which were then negatively bead enriched for CD4+ T cells (Mouse CD4+ T cell Kit, Miltenyi Biotec). 106 CD4+ T cells were resuspended in 1 mL Ca2+ buffer (140mM NaCl, 5mM KCl, 1.2mM CaCl2, 0.5mM MgCl2, 5mM Glucose, 10mM HEPES, pH7.4 with Tris-base), and 1µL Fura-2 and 1µL P-127 solution were added. Cells were incubated for 30 minutes at 37°C and washed in Ca2+ buffer, and then added to an empty well of carbon-coated 8-well chamber slide (Tab-Tek, WUSTL Center for Cellular Imaging). Cells were imaged on a Zeissaxiovert 200M microscope equipped with a Xenon arc lamp, and analyzed using Metamorph (Molecular Devices) as described (2). CD3e/IgG complexes (CD3ε, clone 2C11, Biolegend; Biotin Goat anti-hamster, Biolegend) were added at the 5-minute time point.
2-Photon Microscopy
2-photon imaging was performed using a Leica SP8-2 2-photon microscope, equipped with a Mai Tai HP DeepSee Laser tuned to 900nM and a 25X water-dipping objective. The scale is indicated on the image.
Statistical Analysis
Prism 7 software for Mac OS X was used for all statistical analysis. Statistical significance was determined using the unpaired t-test, and a P value of <0.05 was designated as the criterion for significance.
Results
Generation of a mouse expressing an inducible human voltage-gated sodium channel, Scn5a
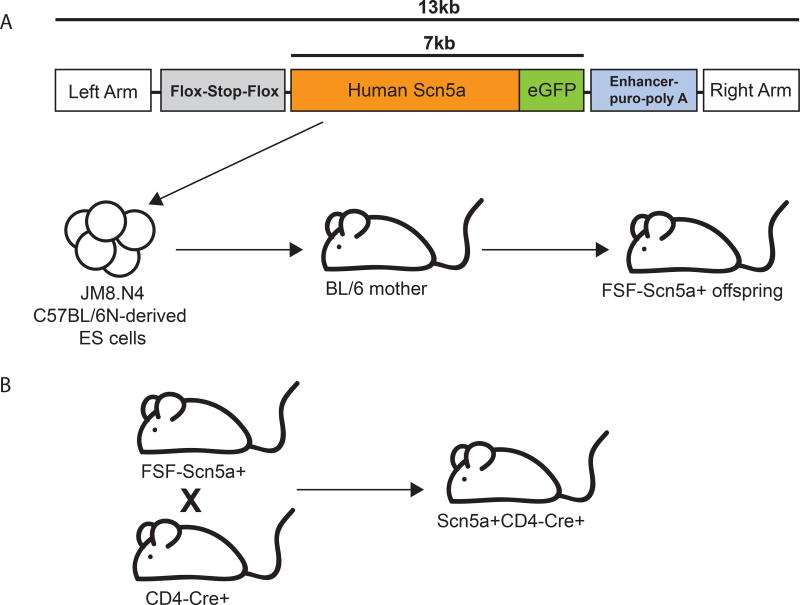
We have previously demonstrated that ectopic expression of human Scn5a is sufficient to endow CD4+ T cell hybridoma cells with the ability to respond to their positively selecting ligand, which normally does not occur due to the very weak nature of this interaction. We wished to further evaluate the role of TCR sensitization in modifying T cell responses in peripheral cells; however, the large size of Scn5a message (6kb+) and our desire to examine the effect in naïve T cells limited our ability to express it in non-transformed cells or by using lentiviruses. To bypass this obstacle, we generated an inducible Scn5a knock-in mouse. To create this mouse, a flox-stop-flox cassette was inserted upstream of the human Scn5a-GFP cDNA, and this construct (driven by a CMV promoter) was knocked into the Rosa 26 locus in C57/BL6 derived ES cells (Figure 1A). The ES cells were used to generate a knock-in mouse line, which was then crossed to a CD4-Cre line to induce expression only in T cells (Figure 1B). Under these circumstances, all αβ T cells should express Scn5a following CD4 upregulation during thymic selection in the resulting Scn5a+CD4-Cre+ mice.
Figure 1. Generation of a mouse expressing an inducible human voltage-gated sodium channel, Scn5a.
(A) To create the Scn5a mice, a flox-stop-flox cassette from pCALSL-mir30 was inserted upstream of the human Scn5a-GFP cDNA, and this construct was targeted to the Rosa26 locus by electroporating into JM8.N4 C57BL/6N-derived ES cells. Two successfully targeted ES clones were injected into blastocysts and both lines were transmitted in the germline. (B) One line was selected to be bred to a CD4-Cre expressing strain, to yield Scn5a+CD4-Cre+ offspring.
We wanted to ensure that our inducible construct was working as expected; however, there are no available antibodies that can detect Scn5a by FACs or immunohistochemistry. We therefore crossed the inducible-Scn5a line to Ert2-Cre, where Cre expression can be induced in all tissues by the administration of tamoxifen (Supplementary Figure 1A). Scn5a is normally expressed at high levels in the myocardium and conductive tissue of the heart, so we reasoned that we could observe high levels of our Scn5a-GFP expression in the heart. We induced the Scn5a gene by gavage of tamoxifen and 24 hours later examined the heart for GFP expression by 2-photon microscopy. Indeed, we readily detected GFP+ cells in the heart in the inducible Scn5a×Ert2-Cre mice, but not in Cre-negative littermates, confirming that our construct was inducible in vivo (Supplementary Figure 1B). We did not detect any GFP+ expression in the T cells in the Scn5a-CD4-Cre mice by FACS, indicating a relatively low level of expression; this low but functional level is consistent with other studies of expression of ion channels in T cells (2, 34).
Expression of human Scn5a under the control of CD4 does not affect mouse T cell development
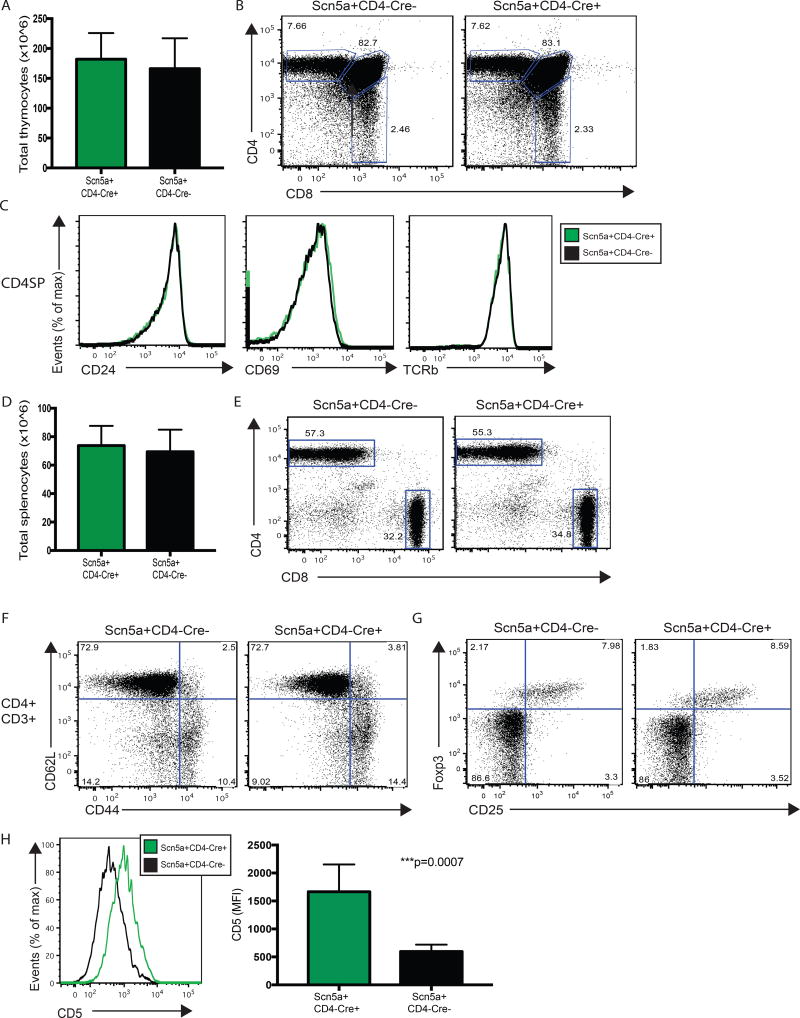
We next wanted to determine if co-expression of human and mouse Scn5a during thymocyte development altered T cell development in the Scn5a+CD4-Cre+ mice, relative to their wild-type littermates. Because Scn5a is endogenously expressed in the thymus, we were not expecting to see any appreciable changes in thymocyte counts and, indeed, we found total thymocyte counts in Scn5a+CD4-Cre+ and Scn5a+CD4-Cre- mice to be equivalent (Figure 2A). The resulting Scn5a+CD4-Cre+ mice also exhibited normal thymocyte development, in terms of CD4/CD8 T cell skewing and expression of early activation and maturity markers, including CD24, CD69, and TCRβ, again, relative to their transgene-negative littermates (Figures 2B and 2C, and Supplementary Figures 2A and 2B). The two cohorts likewise exhibited equivalent numbers of developing CD4+Foxp3+CD25hi thymic regulatory T cells (Supplementary Figure 2C).
Figure 2. T cell development and phenotype is comparable in Scn5a+CD4-Cre+ mice and their Scn5a+CD4-Cre- littermates, except for CD5 expression.
(A) Whole thymus was isolated from 6–8 week old Scn5a+CD4-Cre+ mice (n=5) and Scn5a+CD4-Cre- littermates (n=5). Thymii were processed into single cell suspensions to determine whole thymocyte counts. (B) Single cell thymocyte suspensions from Scn5a+CD4-Cre+ mice and Scn5a+CD4-Cre- littermates were analyzed by FACS to assess thymocyte development. Live/dead gating was followed by doublet discrimination before CD4 and CD8 levels were compared. (C) The CD4+CD8- (CD4 SP) population was assessed for expression levels of CD24, CD69 and TCRβ. (D) Whole spleen was isolated from 6–8 week old Scn5a+CD4-Cre+ mice (n=12) and Scn5a+CD4-Cre- littermates (n=10). Spleens were processed into single cell suspensions and red blood cells were lysed to determine whole splenocyte counts. (E) Single cell splenocyte suspensions from Scn5a+CD4-Cre+ mice and Scn5a+CD4-Cre- littermates were analyzed by FACS to assess the peripheral T cell compartment. Live dead/gating was followed by doublet discrimination. CD3+ cells were then analyzed for CD8+ and CD4+ populations. (F) Within the CD3+CD4+ T cell compartment, antigen experience was assessed using CD44 and CD62L staining. (G) Within the CD3+CD4+ T cell compartment, the presence of Tregs was assessed using Foxp3 and CD25 staining. (H) Within the CD3+CD4+ T cell compartment, CD5 levels were analyzed and compared, using mean fluorescence intensity (MFI).
Splenocyte counts as well as peripheral CD4+ and CD8+ T cell skewing were the same in Scn5a+CD4-Cre+ mice and their wild-type counterparts (Figures 2D and 2E), with equivalent expression of activation markers CD44 and CD62L (Figure 2F and Supplementary Figure 2D). We also observed equal numbers of splenic CD4+Foxp3+CD25hi Tregs (Figure 2G), equal expression of peripheral regulatory molecules CTLA-4 and PD-1 by splenic CD4+ and CD8+ T cells (Supplementary Figures 2F and 2G), and equal production of IL-2 and IFNγ upon ex vivo stimulation (PMA + Ionomycin) of CD4+ and CD8+ T cells (Supplementary Figure 2H). Overall, these data indicate that additional expression of Scn5a via our construct does not affect the development, emigration, or effector capacity of T cells from the thymus.
The only phenotypic difference detected between Scn5a+CD4-Cre+ mice and their wild-type littermates was an increase in the level of surface CD5 expression. Mean fluorescence intensity (MFI) of CD5 increased significantly on both CD4+ and CD8+ peripheral T cells (Figure 2H and Supplementary Figure 2E). The increased expression of CD5 on both CD4+ and CD8+ T cells is consistent with our supposition that expression of Scn5a will increase the sensitivity of T cells to self-pMHC. The Scn5a-CD4-Cre mice appear healthy and show no signs of autoimmune disease, even at ages up to ten months.
TCR sensitization via Scn5a expression correlates with more rapid and robust TCR-proximal signaling
A series of tightly regulated signaling events occur as a result of TCR/co-receptor engagement on the surface of T cells. These events result in phosphorylation/activation of Phospholipase C- gamma (pPLCγ). Active PLCγ then cleaves cell membrane phosphatidylinositol 4,5-bisphosphate (PIP2) to yield the second messengers diacylglycerol (DAG) and inositol triphosphate (IP3), and IP3 in turn binds to gated channels that release highly concentrated Ca2+ stores in the endoplasmic reticulum. The subsequent activation of calcineurin triggered by Ca2+ release leads to dephosphorylation of NFAT, which is released from the cytoplasm to travel to the nucleus, where it then regulates the many and diverse responses of an activated T cell, such as cytokine production. Our previous studies of Scn5a in thymocytes showed that Scn5a increased the magnitude and sustained levels of Ca2+ influx to positive selecting ligands (2).
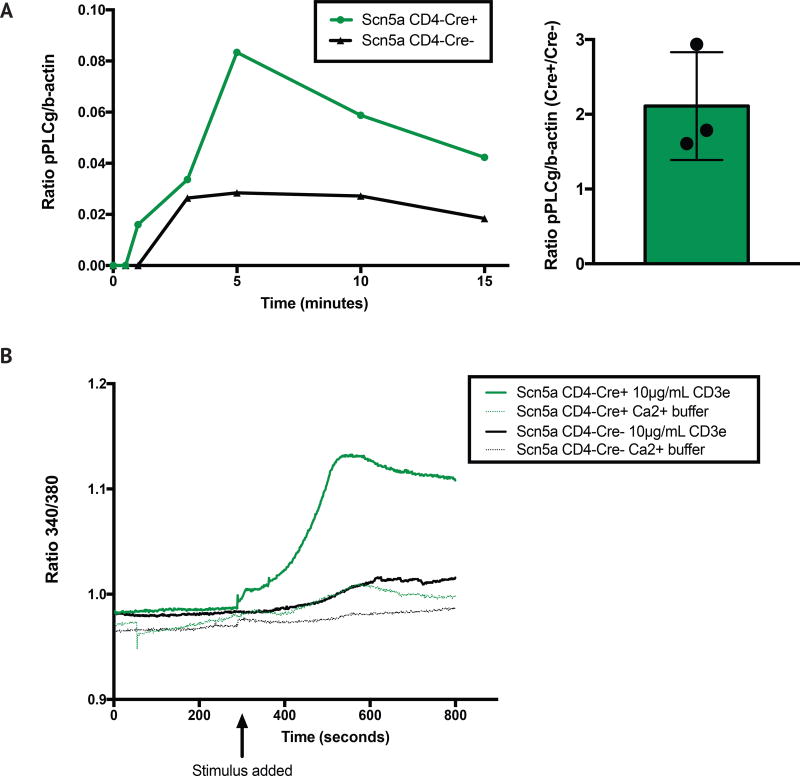
We thus wished to determine if ectopic Scn5a expression in peripheral T cells could lead to appreciable changes in TCR-proximal signaling. To do so, we first measured phosphorylation of PLCγ in response to CD3 crosslinking at multiple time points, in CD4+ T cells from both Scn5a+CD4-Cre+ mice and their Scn5a+CD4-Cre- (“wild-type”) littermates. We found that phosphorylation of PLCγ is more rapid in Scn5a+ mice: it is observable by as soon as 1 minute post-stimulation in Scn5a+CD4-Cre+ mice, but not wild-type littermates, indicating an increased sensitivity to stimulus (Figure 3A). Further, maximum phosphorylation, which occurred at 5 minutes post-stimulation, is approximately 3 times greater in Scn5a+CD4-Cre+ mice. This significant difference is still apparent even as late as 15 minutes post-stimulation (Figure 3A).
Figure 3. TCR sensitization via Scn5a expression correlates with more rapid and robust TCR-proximal signaling, using PLCγ phosphorylation and Ca2+ flux as readouts.
(A) Phosphorylation of PLCγ (relative to b-actin) in response to sub-maximal CD3 crosslinking (10µg/mL) was measured at 30 sec., 1 min., 3 min., 5 min., 10 min. and 15 min., in 1.5×106 CD4+ enriched T naïve splenocytes from both Scn5a+CD4-Cre+ mice and their Scn5a+CD4-Cre- littermates. Fold change (a ratio of Scn5a+CD4-Cre+ to Scn5a+CD4-Cre- pPLCγ:b-actin maximum levels) is quantified on the right. Data are representative of three separate experiments, with splenocytes from two mice pooled within cohorts. (B) Ca2+ flux was measured in 106 CD4+ enriched T naïve splenocytes from both Scn5a+CD4-Cre+ mice and their Scn5a+CD4-Cre- littermates. CD3e/IgG complexes were added at 5 minutes, except in the control wells, in which Ca2+ buffer alone was used. Data are representative of three separate experiments, with splenocytes from two mice pooled within cohorts.
We also examined Ca2+ flux in both cohorts of mice upon TCR crosslinking, and found that low levels of stimulation revealed an increased sensitivity of signaling in Scn5a+CD4-Cre+ mice. At a low, suboptimal dose of 10µg/ml, there is a robust flux apparent in Scn5a+CD4-Cre+ cells, but no measurable response in wild-type cells (Figure 3B). In contrast, at very high levels of stimulation (31.6µg/ml), both cohorts reach a similar peak, although Ca2+ influx rose more quickly in the Scn5a+CD4-Cre+ CD4+ T cells (Supplementary Figure 3). Together, these data indicate that increasing TCR sensitivity in peripheral T cells results in more TCR-proximal signaling that is faster and more robust in nature.
CD4+ T cells with increased sensitivity to self-pMHC have an impaired response to Listeria monocytogenes infection
With the significant changes in TCR-proximal signaling observed in Scn5a+CD4-Cre+ mice, we wanted to determine if there were any in vivo functional consequences of Scn5a expression, in the context of infection. To this end, we crossed Scn5a+CD4-Cre+ mice to our LLO56.Rag1−/− and LLO118.Rag1−/− strains of mice (expressing the Thy1.1 and Ly5.1 congenic markers, respectively), to generate LLO56.Rag1−/−.Thy1.1+/+Scn5a+CD4-Cre+ and LLO118.Rag1−/−Ly5.1+/+Scn5a+CD4-Cre+ mice (hereafter, referred to as LLO56.Scn5a+CD4-Cre+ and LLO118.Scn5a+CD4-Cre+) (Figure 4A).
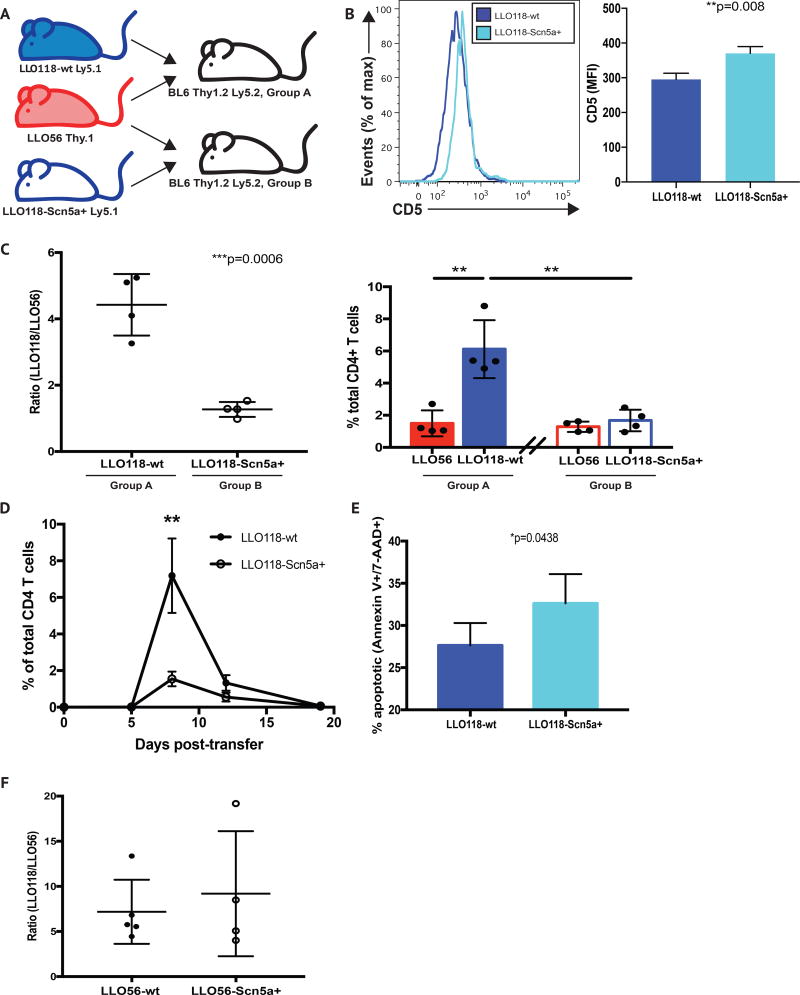
Figure 4. CD4+ T cells expressing the self-sensitizing Scn5a channel have an impaired response to Listeria monocytogenes infection.
(A) Recipient B6 (CD45.2+ and CD90.2+) mice were co-injected with either 104 LLO56 CD90.1+ T cells and 104 LLO118 CD45.1+ T cells (Group A), or 104 LLO56 CD90.1+ T cells and 104 Scn5a-expressing LLO118 CD45.1+ T cells (Group B), on day 0. (B) Within the CD3+CD4+ T cell compartment of wt and Scn5a-expressing LLO118 naive mice, CD5 levels were analyzed and compared, using mean fluorescence intensity (MFI). (C) Mice from Groups A and B were IV injected with 103 CFU L. monocytogenes in PBS on day 1, and mice were sacrificed at day 7. Red blood cell-lysed, single cell splenocyte suspensions from both groups were analyzed by FACS. Live dead/gating was followed by doublet discrimination. CD3+CD4+ cells were then analyzed for the presence of CD45.1+ (LLO118) and CD90.1+ (LLO56) donor cells, and the resulting ratios (left) and absolute percentages (right) of these populations are shown. Data are representative of three separate experiments, with a minimum of 4 mice per group, per experiment. (D) A time course analysis of cell expansion was performed using the same experimental setup described in (C). A minimum of 3 mice from each cohort was analyzed at days 4, 7, 12 and 19 post-infection. (E) Ly5.1-marked cells recovered from donors receiving either wt LLO118 or Scn5a+ LLO118 cells CD4+ T cells were analyzed for the presence of apoptosis at day 7 post-infection, via Annexin V and 7-AAD staining. (F) Recipient B6 (CD45.2+ and CD90.2+) mice were co-injected with either 104 LLO56 CD90.1+ T cells and 104 LLO118 CD45.1+ T cells, or 104 Scn5a-expressing LLO56 CD90.1+ T cells and 104 LLO118 CD45.1+ T cells, on day 0. Both sets of receipients were IV injected with 103 CFU L. monocytogenes in PBS on day 1, and mice were sacrificed at day 7. Red blood cell-lysed, single cell splenocyte suspensions from both groups were analyzed by FACS. Live dead/gating was followed by doublet discrimination. CD3+CD4+ cells were then analyzed for the presence of CD45.1+ (LLO118) and CD90.1+ (LLO56) donor cells, and the resulting ratios are shown. Data are representative of three separate experiments, with a minimum of 4 mice per group, per experiment.
We found total thymocyte counts in LLO56.Scn5a+CD4-Cre+ and CD4-Cre-negative mice, and LLO118.Scn5a+CD4-Cre+ and CD4-Cre-negative mice, to be equivalent (Supplementary Figures 4A and 4J). However, the resulting LLO56.Scn5a+CD4-Cre+ and LLO118.Scn5a+CD4-Cre+ mice exhibited increased numbers of CD4 single positive thymcoytes, with decreases in the corresponding CD4/CD8 DP thymocyte populations (Supplementary Figures 4B, 4C, 4D, 4K, 4L, and 4M). These changes are indicative of stronger positive selection in the thymus, and mirror the differences we observe in our wt LLO56 versus LLO118 mice, where we find more CD4 SP thymocytes in the LLO56 mouse (30). Otherwise, developing thymocytes in the LLO56.Scn5a+CD4-Cre+ and LLO118.Scn5a+CD4-Cre+ exhibit similar levels of early activation and maturity markers, including CD24 and TCRβ, relative to their respective CD4-Cre-negative littermates (Supplementary Figures 4E and 4N).
Splenocyte counts were also the same in LLO56.Scn5a+CD4-Cre+ and CD4-Cre-negative mice, and LLO118.Scn5a+CD4-Cre+ and CD4-Cre-negative mice (Supplementary Figures 4G and 4P), with equivalent expression of activation markers CD44 and CD62L on peripheral CD4+ T cells (Supplementary Figures 4H and 4Q). Further, the expression of Scn5a on the LLO56 and LLO118 backgrounds did not lead to the generation of Tregs in the thymus or the periphery of these mice (data not shown); importantly, Tregs are found in only very negligible numbers in wild-type LLO56 and LLO118 mice (30).
We did not observe differences in CD5 levels between the developing thymocytes of LLO56.Scn5a+CD4-Cre+ and CD4-Cre-negative mice, and LLO118.Scn5a+CD4-Cre+ and CD4-Cre-negative mice, nor did we find differences in CD5 levels when comparing mature peripheral CD4+ T cells from LLO56.Scn5a+CD4-Cre+ and CD4-Cre-negative mice (Supplementary Figures 4F, 4I and 4O). However, we did observe an upregulation of CD5 in splenic CD4+ T cells from LLO118.Scn5a+CD4-Cre+ mice, relative to their CD4-Cre-negative littermates (Figure 4B).
Using an experimental setup previously characterized in the lab, recipient B6 (Ly5.2+ and Thy1.2+) mice were co-injected with either 104 LLO56 T cells and 104 LLO118 (“LLO118 wild-type”) T cells, or 104 LLO56 T cells and 104 LLO118.Scn5a+CD4-Cre+ T cells, on day 0 (30). Mice were then infected with 103 CFU L. monocytogenes on day 1, and splenocytes were examined 7 days later. While LLO118 wild-type T cells were recovered in roughly a 5:1 ratio (relative to LLO56 cells) in the spleens of recipient mice, LLO118.Scn5a+CD4-Cre+ were found at a much lower frequency, at close to a 1:1 ratio with LLO56 cells (Figure 4C). This indicated a decreased primary response of the highly sensitive LLO118.Scn5a+Cre-CD4+ cells to L. monocytogenes infection. There was no change in the LLO56 response, as the relative frequency of these cells remained the same in the two cohorts (Figure 4C).
To examine this altered response in greater detail, we set up an infection time course in which we analyzed CD4+ T cells (LLO118.Scn5a+CD4-Cre+ and LLO118 wild0-type) at days 4, 12 and 19 post-infection, in addition to our standard day 7 harvest. We found no difference in cell numbers at day 4, and although we did observe trending differences in cell numbers at day 12 (more LLO118 wild-type CD4+ T cells present in the spleen), these differences did not reach statistical significance (Figure 4D). By day 19 both CD4+ T cell populations had contracted to similarly low numbers (Figure 4D). These results parallel the findings in our original LLO56/LLO118 transfer system, where we see the difference in LLO56 and LLO118 frequency disappear during “resting” time points (30). To better understand the dynamics of these cells, we also analyzed cells recovered at day 7 post-infection for signs of apoptosis. We recovered significantly more apoptotic (Annexin V+/7-AAD+) Ly5.1+ CD4+ T cells from the recipients of LLO118.Scn5a+CD4-Cre+ cells, compared to recipients that received cells from LLO118 wild-type donors (Figure 4E). Therefore, it appears that the deficiency in numbers of LLO118.Scn5a+CD4-Cre+ cells at d7 is due at least in part to increased apoptosis.
Using a similar transfer system, responses of LLO56.Scn5a-to L. monocytogenes infection were also examined. In this case, recipient B6 mice were co-injected with either 104 LLO56 T cells (“LLO56 wild-type”) and 104 LLO118 T cells, or 104 LLO56.Scn5a+CD4-Cre+ T cells and 104 LLO118 T cells, on day 0. When these infected transfers were examined at day 7, we saw no change in the relative frequency of LLO56.Scn5a+CD4-Cre+ cells (Figure 4F). This indicated that LLO56 signaling sensitivity is already at such a level that additional TCR sensitization via Scn5a expression leads to no further change in response. These data show that the impact of ectopic Scn5a expression in peripheral T cells appears to be limited to increases in proximal TCR signaling, with a compensatory decreased in vivo response.
Discussion
In this report, we have described the production and characterization of Scn5a VGSC-transgenic mice. On a polyclonal background, T cells from these mice express increased levels of surface CD5, indicating that their TCRs have experienced elevated perception of self-peptide; otherwise, these cells are phenotypically identical to T cells from wild-type littermates, with no alterations in T cell development, skewing, or maturation. We also report that TCR proximal signaling is more sensitive and vigorous in Scn5a-transgenic CD4+ T cells. In spite of this increase in TCR proximal signaling, we find the cellular response to Listeria monocytogenes infection to be impaired when Scn5a transgene-expressing mice are bred to a TCR transgenic recognizing listerolysin O (LLO118). In fact, cells from the sensitized LLO118.Scn5a+ mouse resemble those from the CD5hi, highly self-reactive LLO56 in their response to infection. From these data, we conclude that there is a crucial relationship between TCR:self-peptide interaction and the response of T cells to infection - namely, that there is an inverse relationship between TCR sensitivity to self and the capacity for clonal expansion during a primary immune response.
Importantly, it appears that the effects of peripheral Scn5a expression are limited to proximal TCR signaling, with no effect on homeostasis or survival. This argument is supported by the observations that a) the resting phenotypes of Scn5a+CD4-Cre+ thymic and peripheral T cells are the same as those in wild-type littermates, aside from CD5 expression, and b) we see a differing in vivo impact when Scn5a is peripherally expressed in LLO118 cells, which are minimally self-reactive, versus LLO56 cells, which are highly self-reactive. If the effect of ectopic Scn5a expression were simply a broad dampening of cellular activity, we would expect to see an impaired response from the both the LLO118.Scn5a+ and LLO56.Scn5a+ mouse; we did not find this to be the case in the latter.
Although the number of positively selected thymocytes increases in LLO TCR transgenic lines with Scn5a expression, we do not observe changes in CD5 levels (or levels of other developmental markers) on the developing thymocytes. However, we do observe increased levels of CD5 on peripheral polyclonal Scn5a+ CD4+ T cells and peripheral LLO118.Scn5a+ CD4+ T cells. This distinction implies that ectopic Scn5a expression has more of an influence on peripheral T cell function than on T cell development itself, during which Scn5a is normally expressed.
Scn5a is normally only expressed in T cells until the SP stage of thymocyte development. However, here we utilize the peripheral expression of Scn5a as a means of modifying TCR sensitivity and signaling in peripheral T cells, beyond the scope of T cell development in the thymus. Importantly, we don’t intend to imply any role for this VGSC channel per se in the periphery outside these conditions of altered expression. Rather, in our system, Scn5a expression is used as a way to examine self-reactivity. Tonic signaling, an important aspect of T cell maintenance, has historically been hard to manipulate and examine without great alteration of the immune response, as in the case of mutation or deletion of key signaling molecules, for example (35, 36). Our VGSC transgenic approach thus has the advantage of leaving key signaling intermediates in their intact, naturally occurring states.
To this end, these studies have revealed that the level of tonic signaling in a T cell is deterministic for the magnitude of its response to a primary infection. Through use of Scn5a expression, we have demonstrated this directly by increasing the sensitivity of TCR signaling, and in turn finding a corresponding decrease in the in vivo response. The presence of Scn5a necessarily sensitizes developing T cells in the thymus, due to the fact that self-p:MHC signaling there is otherwise very weak. Along with our collective data, this observation raises the question: must Scn5a (and other such thymic VGSC components) be “switched off” to appropriately dampen T cell responses in the periphery, and help prevent unwanted autoimmunity? The role of other voltage-gated channels in T cell development and function has been widely studied and reviewed, and there is mounting evidence for the use of selective potassium and calcium channel inhibitors in the treatment of autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and irritable bowel syndrome (34, 37, 38). The relationship between T cell activation and VGSC signaling is a nuanced one and has not been well studied in the context of autoimmunity, but it seems that further manipulation and analysis of these tightly expressed channels could give great insight into the genesis of inflammation and autoimmunity.
Importantly, the finding that CD5 levels on resting polyclonal CD4+ T cells and LLO118 CD4+ T cells increased with peripheral expression of Scn5a is a significant one, as we cannot rule out the possibility that the dampened in vivo response of the LLO118.Scn5a cells is due in part to the immunomodulatory effects of CD5 itself. Indeed, the role of CD5 in primary T cell function is not firmly established. CD5 was originally considered to be a co-stimulatory molecule because antibodies to it were found to potentiate T cell activation. However, thymocytes from CD5-knockout mice show enhanced - not reduced - proliferation to TCR stimulation, establishing that CD5 functions as a negative regulator (24). CD5 is well-documented negative regulator of both T and B lymphocytes (19–23, 25, 39, 40), but it has also been demonstrated that there is a role for CD5 in lymphocyte survival (21, 41).
It has been proposed that CD5 expression helps a T cell to tune its threshold of T cell activation, to prevent negative selection (13, 18, 25, 42), and that interaction with cellular regulators such as SHP-1, Ras-GAP, and c-Cbl is (at least in part) responsible for immune modulation (21, 43, 44). CD5 blocks mTOR activation induced by cytokines, thereby facilitating Treg induction (45). The cytoplasmic domain of CD5 is phosphorylated upon T cell activation and contains an ITIM domain that can associate with the SHP-1 phosphatase. However, the CD5 inhibitory activity is not affected by SHP-1 knockout in T cells (42). Thus, it is still unclear how CD5 functions as an effector molecule.
Other groups have reported that CD5hi cells proliferate more robustly in a primary response, compared to CD5lo cells (46, 47). Our laboratory, on the other hand, has found that CD5lo T cells respond more vigorously in a primary response (30). The explanation for these differences is not established, but factors could include differences in the form or strength of antigen, limited number of T cells examined, or effects of microbiota on immune responses. Despite these unresolved differences, however, is a consensus that CD5 is an excellent marker for self-reactivity of a T cell. Whatever the exact role CD5 plays in the response of LLO118.Scn5a cells to infection, our data demonstrates a direct link between TCR sensitivity to self and cell fate during infection.
On the whole, the outcomes described herein could also be harnessed to potentially enhance T cell responses in vivo. For instance, Stronen and colleagues have recently described the use of naive donor-derived T cells for targeting cancer neoantigens (48). In such a scenario, it might be beneficial to enrich donor populations for “CD5lo expressors”, to improve the chances of transferring T cells with greater potential for responding to tumor antigens. There has already begun a movement toward selective enrichment of T cells for improving adoptive immunotherapy (49–51). In further support of this notion, Dorothée and colleageus showed, via an analysis of circulating T cell clones, that cytolytic antitumor activity was inversely proportional to CD5 levels (52). They too suggested the intriguing possibility of using CD5 tuning as a method of enhancing T cell infiltration of tumors, and future studies to this effect therefore seem highly justified.
Supplementary Material
Acknowledgments
Funding
This work was supported by National Institutes of Health Research Grants AI24157 and 126150 to PMA.
Footnotes
Author Contributions
AVM designed and performed experiments and wrote the manuscript; JMB, DLD and SH performed experiments; VD, SR, HY and KMM created the Scn5a-GFP construct and the targeted ES cells; VR and BZ performed 2 photon microscopy; JMW derived the Scn5a mice; and PMA designed experiments and wrote the manuscript.
References
- 1.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo WL, Donermeyer DL, Allen PM. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat. Immunol. 2012;13:880–887. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat. Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer E. Negative selection--clearing out the bad apples from the T-cell repertoire. Nat. Rev. Immunol. 2003;3:383–391. doi: 10.1038/nri1085. [DOI] [PubMed] [Google Scholar]
- 5.Germain RN. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2002;2:309–322. doi: 10.1038/nri798. [DOI] [PubMed] [Google Scholar]
- 6.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 7.Caton AJ, Cozzo C, Larkin J, 3rd, Lerman MA, Boesteanu A, Jordan MS. CD4(+) CD25(+) regulatory T cell selection. Ann. N. Y. Acad. Sci. 2004;1029:101–114. doi: 10.1196/annals.1309.028. [DOI] [PubMed] [Google Scholar]
- 8.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunol. Cell Biol. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 9.Stritesky GL, Jameson SC, Hogquist KA. Selection of self-reactive T cells in the thymus. Annu. Rev. Immunol. 2012;30:95–114. doi: 10.1146/annurev-immunol-020711-075035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin B, Auffray C, Delpoux A, Pommier A, Durand A, Charvet C, Yakonowsky P, de Boysson H, Bonilla N, Audemard A, Sparwasser T, Salomon BL, Malissen B, Lucas B. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nat Commun. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- 11.Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nat. Rev. Immunol. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- 12.Kirberg J, Berns A, von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J. Exp. Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17:425–436. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 14.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 15.Myers DR, Lau T, Markegard E, Lim HW, Kasler H, Zhu M, Barczak A, Huizar JP, Zikherman J, Erle DJ, Zhang W, Verdin E, Roose JP. Tonic LAT-HDAC7 Signals Sustain Nur77 and Irf4 Expression to Tune Naive CD4 T Cells. Cell Rep. 2017;19:1558–1571. doi: 10.1016/j.celrep.2017.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markegard E, Trager E, Yang CW, Zhang W, Weiss A, Roose JP. Basal LAT-diacylglycerol-RasGRP1 signals in T cells maintain TCRalpha gene expression. PLoS One. 2011;6:e25540. doi: 10.1371/journal.pone.0025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers DR, Zikherman J, Roose JP. Tonic Signals: Why Do Lymphocytes Bother? Trends Immunol. 2017 doi: 10.1016/j.it.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brossard C, Semichon M, Trautmann A, Bismuth G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J. Immunol. 2003;170:4623–4629. doi: 10.4049/jimmunol.170.9.4623. [DOI] [PubMed] [Google Scholar]
- 20.Pena-Rossi C, Zuckerman LA, Strong J, Kwan J, Ferris W, Chan S, Tarakhovsky A, Beyers AD, Killeen N. Negative regulation of CD4 lineage development and responses by CD5. J. Immunol. 1999;163:6494–6501. [PubMed] [Google Scholar]
- 21.Soldevila G, Raman C, Lozano F. The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Curr. Opin. Immunol. 2011;23:310–318. doi: 10.1016/j.coi.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raman C. CD5, an important regulator of lymphocyte selection and immune tolerance. Immunol. Res. 2002;26:255–263. doi: 10.1385/IR:26:1-3:255. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Villar JJ, Whitney GS, Bowen MA, Hewgill DH, Aruffo AA, Kanner SB. CD5 negatively regulates the T-cell antigen receptor signal transduction pathway: involvement of SH2-containing phosphotyrosine phosphatase SHP-1. Mol. Cell. Biol. 1999;19:2903–2912. doi: 10.1128/mcb.19.4.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Muller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995;269:535–537. doi: 10.1126/science.7542801. [DOI] [PubMed] [Google Scholar]
- 25.Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J. Immunol. 2001;166:5464–5472. doi: 10.4049/jimmunol.166.9.5464. [DOI] [PubMed] [Google Scholar]
- 26.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science. 1996;274:1906–1909. doi: 10.1126/science.274.5294.1906. [DOI] [PubMed] [Google Scholar]
- 27.McAlister MS, Brown MH, Willis AC, Rudd PM, Harvey DJ, Aplin R, Shotton DM, Dwek RA, Barclay AN, Driscoll PC. Structural analysis of the CD5 antigen--expression, disulphide bond analysis and physical characterisation of CD5 scavenger receptor superfamily domain 1. Eur. J. Biochem. 1998;257:131–141. doi: 10.1046/j.1432-1327.1998.2570131.x. [DOI] [PubMed] [Google Scholar]
- 28.Davies AA, Ley SC, Crumpton MJ. CD5 is phosphorylated on tyrosine after stimulation of the T-cell antigen receptor complex. Proc. Natl. Acad. Sci. U. S. A. 1992;89:6368–6372. doi: 10.1073/pnas.89.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burgess KE, Yamamoto M, Prasad KV, Rudd CE. CD5 acts as a tyrosine kinase substrate within a receptor complex comprising T-cell receptor zeta chain/CD3 and protein-tyrosine kinases p56lck and p59fyn. Proc. Natl. Acad. Sci. U. S. A. 1992;89:9311–9315. doi: 10.1073/pnas.89.19.9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber KS, Li QJ, Persaud SP, Campbell JD, Davis MM, Allen PM. Distinct CD4+ helper T cells involved in primary and secondary responses to infection. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9511–9516. doi: 10.1073/pnas.1202408109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graw F, Weber KS, Allen PM, Perelson AS. Dynamics of CD4(+) T cell responses against Listeria monocytogenes. J. Immunol. 2012;189:5250–5256. doi: 10.4049/jimmunol.1200666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuda T, Cepko CL. Controlled expression of transgenes introduced by in vivo electroporation. Proc. Natl. Acad. Sci. U. S. A. 2007;104:1027–1032. doi: 10.1073/pnas.0610155104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettitt SJ, Liang Q, Rairdan XY, Moran JL, Prosser HM, Beier DR, Lloyd KC, Bradley A, Skarnes WC. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat Methods. 2009;6:493–495. doi: 10.1038/nmeth.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231:59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, Richelme S, Locksley RM, Aguado E, Malissen M, Malissen B. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Shen S, Chuck MI, Zhu M, Fuller DM, Yang CW, Zhang W. The importance of LAT in the activation, homeostasis, regulatory function of T cells. J. Biol. Chem. 2010;285:35393–35405. doi: 10.1074/jbc.M110.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012;12:532–547. doi: 10.1038/nri3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bittner S, Meuth SG. Targeting ion channels for the treatment of autoimmune neuroinflammation. Ther. Adv. Neurol. Disord. 2013;6:322–336. doi: 10.1177/1756285613487782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persaud SP, Donermeyer DL, Weber KS, Kranz DM, Allen PM. High-affinity T cell receptor differentiates cognate peptide-MHC and altered peptide ligands with distinct kinetics and thermodynamics. Mol. Immunol. 2010;47:1793–1801. doi: 10.1016/j.molimm.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarakhovsky A, Muller W, Rajewsky K. Lymphocyte populations and immune responses in CD5-deficient mice. Eur. J. Immunol. 1994;24:1678–1684. doi: 10.1002/eji.1830240733. [DOI] [PubMed] [Google Scholar]
- 41.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J. Immunol. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong B, Somani AK, Love PE, Zheng X, Chen X, Zhang J. CD5-mediated inhibition of TCR signaling proceeds normally in the absence of SHP-1. Int. J. Mol. Med. 2016;38:45–56. doi: 10.3892/ijmm.2016.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demydenko D. c-Cbl mediated ubiquitylation and regulation of cell surface exposure of CD5. Biochem. Biophys. Res. Commun. 2010;392:500–504. doi: 10.1016/j.bbrc.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 44.Dennehy KM, Broszeit R, Ferris WF, Beyers AD. Thymocyte activation induces the association of the proto-oncoprotein c-cbl and ras GTPase-activating protein with CD5. Eur. J. Immunol. 1998;28:1617–1625. doi: 10.1002/(SICI)1521-4141(199805)28:05<1617::AID-IMMU1617>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 45.Henderson JG, Opejin A, Jones A, Gross C, Hawiger D. CD5 instructs extrathymic regulatory T cell development in response to self and tolerizing antigens. Immunity. 2015;42:471–483. doi: 10.1016/j.immuni.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 46.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC. The TCR's sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat. Immunol. 2015;16:107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, Donia M, Boschen ML, Lund-Johansen F, Olweus J, Schumacher TN. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science. 2016;352:1337–1341. doi: 10.1126/science.aaf2288. [DOI] [PubMed] [Google Scholar]
- 49.Larbi A, Fulop T. From "truly naive" to "exhausted senescent" T cells: when markers predict functionality. Cytometry A. 2014;85:25–35. doi: 10.1002/cyto.a.22351. [DOI] [PubMed] [Google Scholar]
- 50.Klebanoff CA, Gattinoni L, Restifo NP. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? J. Immunother. 2012;35:651–660. doi: 10.1097/CJI.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat. Med. 2017;23:18–27. doi: 10.1038/nm.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorothee G, Vergnon I, El Hage F, Le Maux Chansac B, Ferrand V, Lecluse Y, Opolon P, Chouaib S, Bismuth G, Mami-Chouaib F. In situ sensory adaptation of tumor-infiltrating T lymphocytes to peptide-MHC levels elicits strong antitumor reactivity. J. Immunol. 2005;174:6888–6897. doi: 10.4049/jimmunol.174.11.6888. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.