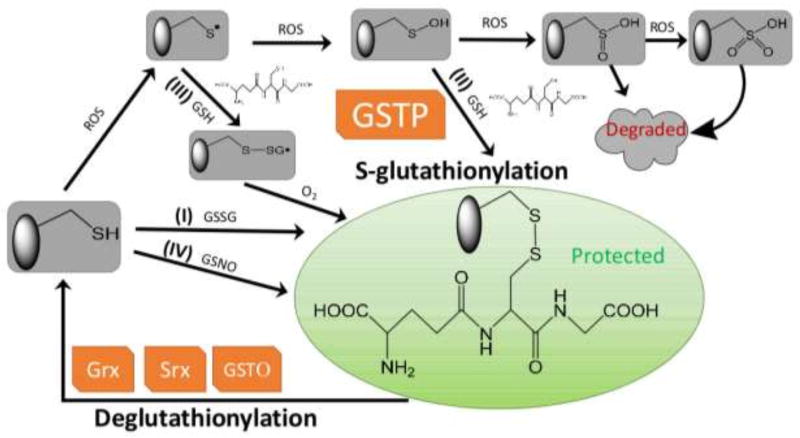
Figure 1.
Protein S-glutathionylation and deglutathionylation cycle. Protein S-glutathionylation proceeds either spontaneously or enzymatically. The non-enzymatic reaction can proceed as follows: (1) thiol-disulfide exchange between protein thiol (PSH) and glutathione disulfide (GSSG); (2) PSH is oxidized by reactive oxygen species (ROS) to a sulfenic acid (PSOH) which then rapidly reacts with GSH to form PSSG, thus prevent the target protein from over-oxidation to sulfinic (SO2H) and eventually sulfonic acid (SO3H) which generally irreversibly deactivates the protein; (3) PSH is oxidized to a thiyl radical (PS•) which rapidly reacts with GSH to form a thiyl radical glutathionyl intermediate (PSSG•−) which can then react with O2 to form PSSG; (4) PSH may be modified by GSNO to form PSNO and/or PSSG. Protein S-glutathionylation can occur spontaneously, but the rates and magnitude are greatly enhanced by catalytic activity of GSTP. Following a forward reaction dependent on GSTP, deglutathionylation can be achieved by Grx, Srx or GSTO.