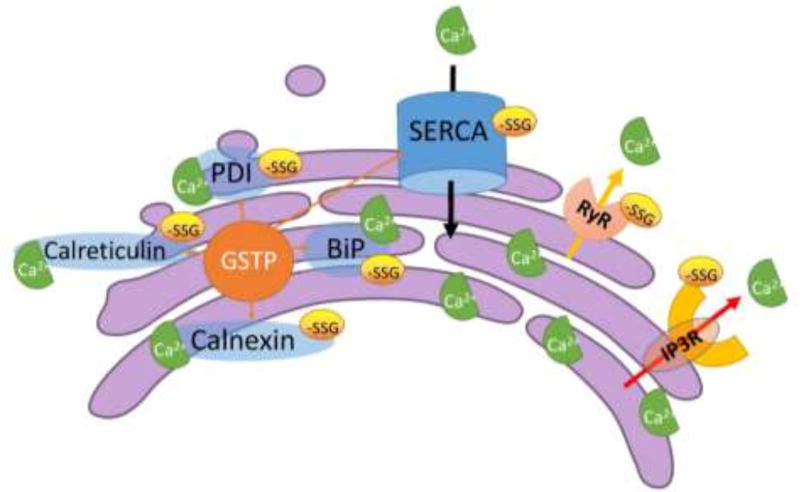
Figure 3.
S-glutathionylation targets in sarco/endoplasmic reticulum (SR/ER). The maintenance of the high Ca2+ concentrations in the SR/ER is primarily achieved by crosstalk between (1) molecular chaperones that bind and buffer Ca2+; (2) Ca2+ pumps that use ATP to work against electrochemical gradients; (3) channel pores that leak or release from SR/ER to the cytosol. Alteration in this balance have the potential to perturb ER proteostasis, resulting in ER stress and the induction of UPR. The effects of S-glutathionylation on Ca2+ release from SR ryanodine receptors (RyRs) and ER inositol 1,4,5-triphosphate receptors (IP3Rs), and on Ca2+ reuptake by sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), have been well reported. The activities of the molecular chaperones in the ER, e.g. calnexin, calreticulin, GRP78/BiP, and PDI, that bind and buffer Ca2+ are also affected by S-glutathionylation. The specific S-glutathionylation sites of each individual protein and the effect of S-glutathionylation on the protein function are discussed in the text. A proportion of the cellular content of GSTP is found in the ER compartment, substantiating the concept that S-glutathionylation may be a crucial regulatory process in the ER.