Abstract
Harmful blooms of domoic acid (DA)-producing algae are a problem in oceans worldwide. DA is a potent glutamate receptor agonist that can cause status epilepticus and in survivors, temporal lobe epilepsy. In mice, one-time low-dose in utero exposure to DA was reported to cause hippocampal damage and epileptiform activity, leading to the hypothesis that unrecognized exposure to DA from contaminated seafood in pregnant women can damage the fetal hippocampus and initiate temporal lobe epileptogenesis. However, development of epilepsy (i.e., spontaneous recurrent seizures) has not been tested. In the present study, long-term seizure monitoring and histology was used to test for temporal lobe epilepsy following prenatal exposure to DA. In Experiment One, the previous study’s in utero DA treatment protocol was replicated, including use of the CD-1 mouse strain. Afterward, mice were video-monitored for convulsive seizures from 2–6 months old. None of the CD-1 mice treated in utero with vehicle or DA was observed to experience spontaneous convulsive seizures. After seizure monitoring, mice were evaluated for pathological evidence of temporal lobe epilepsy. None of the mice treated in utero with DA displayed the hilar neuron loss that occurs in patients with temporal lobe epilepsy and in the mouse pilocarpine model of temporal lobe epilepsy. In Experiment Two, a higher dose of DA was administered to pregnant FVB mice. FVB mice were tested as a potentially more sensitive strain, because they have a lower seizure threshold, and some females spontaneously develop epilepsy. Female offspring were monitored with continuous video and telemetric bilateral hippocampal local field potential recording at 1–11 months old. A similar proportion of vehicle- and DA-treated female FVB mice spontaneously developed epilepsy, beginning in the fourth month of life. Average seizure frequency and duration were similar in both groups. Seizure frequency was lower than that of positive-control pilocarpine-treated mice, but seizure duration was similar. None of the mice treated in utero with vehicle or DA displayed hilar neuron loss or intense mossy fiber sprouting, a form of aberrant synaptic reorganization that develops in patients with temporal lobe epilepsy and in pilocarpine-treated mice. FVB mice that developed epilepsy (vehicle- and DA-treated) displayed mild mossy fiber sprouting. Results of this study suggest that a single subconvulsive dose of DA at mid-gestation does not cause temporal lobe epilepsy in mice.
Keywords: temporal lobe epilepsy, domoic acid, in utero, hilar neurons, Timm stain, FVB mice
1. Introduction
Epilepsy occurs in one of twenty six people (Hesdorffer et al., 2011), and temporal lobe epilepsy is one of the most common types (Engel et al., 1997). In patients with temporal lobe epilepsy, seizures usually start in the hippocampus (Quesney, 1986), which displays characteristic pathology, including hilar neuron loss (Margerison and Corsellis, 1966) and granule cell axon (mossy fiber) sprouting (Sutula et al., 1989; de Lanerolle et al., 1989; Houser et al., 1990; Babb et al., 1991). Temporal lobe epilepsy in patients typically develops after a latent period of years following a brain insult, usually early in life (French et al., 1993). Many initiating brain insults involve prolonged seizures (Mathern et al., 1995). Prolonged seizures can be excitotoxic and cause permanent brain damage (Meldrum and Brierley, 1973).
Temporal lobe epilepsy occurs in other species too. For example, it has been proposed as a common cause of epilepsy in cats (Kitz et al., 2017). Temporal lobe epilepsy can be generated in laboratory rodents by causing prolonged seizures with convulsant drugs, including kainic acid (Hellier et al., 1998) and pilocarpine (Cavalheiro et al., 1996). After recovering from acute seizures, rodents develop spontaneous recurrent seizures that appear to begin in the hippocampus (Toyoda et al., 2013). They also display neuropathology similar to that found in human patients, including hallmark hilar neuron loss and mossy fiber sprouting (Nadler et al., 1980).
In some cases of human temporal lobe epilepsy, no precipitating cause is identified (French et al., 1993; Mathern et al., 1995). It has been proposed that unrecognized exposure to domoic acid from slightly contaminated seafood in pregnant women damages the fetal hippocampus and initiates temporal lobe epileptogenesis (Stewart, 2010). Evidence summarized below is consistent with this hypothesis, but important questions persist.
Domoic acid (DA) is produced by oceanic phytoplankton (Wright et al., 1989; Garrison et al., 1992), and consumption of DA-contaminated seafood can cause seizures and death (Perl et al., 1989; Scholin et al., 2000). Some survivors of DA toxicosis develop permanent anterograde amnesia (Teitelbaum et al., 1990) and temporal lobe epilepsy (Cendes et al., 1995). Adult California sea lions develop temporal lobe epilepsy after DA toxicosis (Scholin et al., 2000; Goldstein et al., 2008; Buckmaster et al., 2014), and in utero exposure has been hypothesized to cause epilepsy to develop in young sea lions (Ramsdell and Zabka, 2008; Ramsdell and Gulland, 2014). Systemically administered DA in rodents causes the development of spontaneous recurrent seizures (Muha and Ramsdell, 2011) and neuropathology similar to human temporal lobe epilepsy (Colman et al., 2005).
DA can cross the placenta, accumulate in amniotic fluid, and enter the fetal brain (Maucher Fuquay et al., 2012). DA is a potent ligand of kainate-type glutamate receptors (Stewart et al., 1990) that are strongly expressed in the hippocampus from early in development. In the rat hippocampus, for which data are available, kainate receptor genes are expressed by E14 and high-affinity kainate binding sites are evident shortly thereafter (Bahn et al., 1994). DA might affect brain development by exciting neurons (Zaczek and Coyle, 1982) and altering gene expression (Hiolski et al., 2014). In utero exposure to DA alters hippocampal connectivity (Mills et al., 2016) and induces persistent neurobehavioral effects (Levin et al., 2005; Tanemura et al., 2009; Zuloaga et al., 2016; Shiotani et al., 2017). In ovo exposure to DA increases seizure susceptibility of larval zebrafish (Tiedeken and Ramsdell, 2007).
Dakshinamurti et al. (1993) reported that a single subconvulsive dose of DA at mid-gestation in mice produces profound impairment in hippocampal function and morphology. Impairments included progressive epileptiform hippocampal EEG activity, progressive death of hippocampal neurons, and enhanced kainate receptor binding in hippocampal synaptosomal membranes (consistent with mossy fiber sprouting). These findings might have relevance to human temporal lobe epilepsy, but the authors acknowledged that longer observations were necessary to test whether clinical seizures actually develop.
The present study employed the single subconvulsive in utero DA treatment protocol of Dakshinamurti et al. (1993) to test for seizures and related neuropathology of temporal lobe epilepsy in mice. Harmful algal blooms are a growing problem (Anderson et al., 2002; Bejarano et al., 2008; Wells et al., 2015), resulting in DA concentrations 1000 times regulatory limits off the Pacific coast of North America in 2015 (McCabe et al., 2016). Although government agencies regulate levels in seafood (Iverson and Truelove, 1994), DA neurotoxicity may occur at lower concentrations in developing nervous systems than adults (Costa et al., 2010). Thus, concerns about toxicity persist, including that undetected low-dose in utero exposure may have detrimental long-term consequences (Potera, 2006; Erdner et al., 2008; Grant et al., 2010; Lefebvre and Robertson, 2010; Pérez-Gómez and Tasker, 2014; Tasker, 2016). It is important to determine if in utero exposure to subconvulsive doses of DA causes temporal lobe epilepsy as DA exposure during pregnancy would be a preventable epileptogenic mechanism.
2. Materials and Methods
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, were approved by a Stanford University institutional animal care and use committee, and comply with ARRIVE guidelines. Mice were housed in micro-isolator cages on wood shavings in groups of up to five. Cotton nestlets were provided for enrichment. Room lights were on from 7:00 a.m. till 7:00 p.m. Mice had unlimited access to rodent chow and water.
2.1 Experiment One
2.1.1 DA treatment
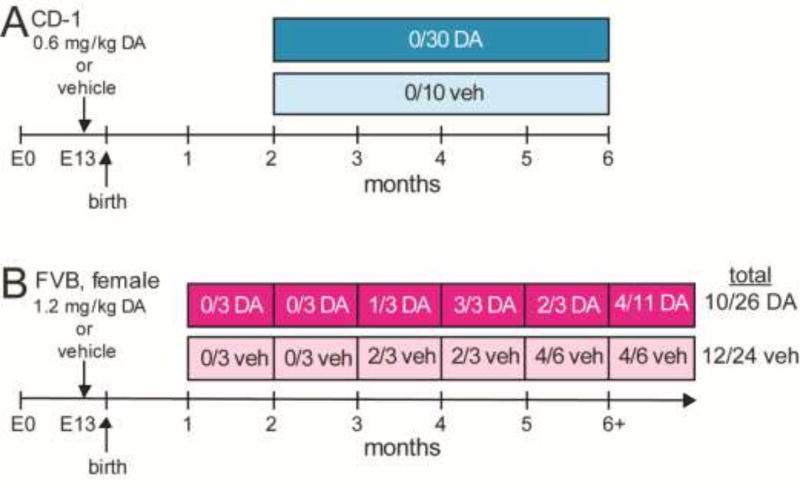
The DA treatment protocol of Dakshinamurti et al. (1993) was replicated (Figure 1A). Lyophilized DA (BioVectra) was reconstituted in vehicle (sterile 0.05 M phosphate buffered saline). Nulliparous CD-1 mice (Charles River) were exposed to 0.6 mg/kg DA by administering 0.29 ml of 0.1 mg/ml solution by tail vein injection on gestation day 13 (evidence of plug at E0). Pregnant mice were observed for convulsive seizures for 2 h after treatment. Offspring were weaned at 23 d old.
Figure 1.
Experimental design. A Experiment One. None of the CD-1 mice exposed in utero to domoic acid (DA) or vehicle (veh) were observed to experience spontaneous convulsive seizures during video-monitoring. Values represent the number of mice that developed epilepsy over number monitored. B Experiment Two. Beginning at one month old, at least three DA-treated and three vehicle-treated female FVB mice were continuously video-EEG monitored for one month periods.
2.1.2 Video seizure monitoring
In mouse models of temporal lobe epilepsy, over 85% of seizures involve motor convulsions that can be detected visually (Buckmaster and Lew, 2011; Mazzuferi et al., 2012; Hester and Danzer, 2013). Beginning at 2 months old, mice were video-recorded for 9 h/d (approximately 8:00 a.m. – 5:00 p.m.) every day until they were 6 months old. Video recordings were reviewed in the fast-forward playback setting for convulsive seizures of stage 3 (forelimb clonus) or greater (Racine, 1972).
2.2 Experiment Two
2.2.1 DA treatment
Lyophilized DA (BioVectra) was reconstituted in vehicle (0.9% bacteriostatic sodium chloride). Nulliparous FVB mice (Charles River) were exposed to 1.2 mg/kg DA by administering 0.16–0.21 ml of 0.2 mg/ml solution by tail vein injection on gestation day 13. Pregnant mice were observed for convulsive seizures for 2 h after treatment.
2.2.2 Electrode implantation
Female offspring were weaned at 23 d old. Beginning at one month of age, mice were randomly chosen, anesthetized with 3% isoflurane, and placed on a temperature-controlled heating pad in a stereotaxic frame. They received antibiotic (enrofloxacin, 10 mg/kg, s.c.), analgesic (carprofen, 5 mg/kg, s.c.), and lactated Ringer’s (1 ml, s.c.). Bupivacaine (0.25%) was infiltrated along the incision site on the scalp. Using aseptic technique, a wireless EEG transmitter (Epoch) was surgically implanted. Recording electrodes (teflon-insulated platinum-iridium, 127 µm diameter) were placed bilaterally in the dorsal hippocampi at 2.2 mm posterior to bregma, 2.5 mm lateral of midline, and 2.0 mm deep. A reference electrode was placed in the frontal cortex. The implant was fixed to the skull with cyanoacrylate, cranioplastic cement, and a jeweler’s screw.
2.2.3 Video-EEG seizure monitoring
One week after surgery, each mouse was placed in an individual cage on a receiver unit (Epoch) and recorded continuously for one month (Figure 1B). Individuals could not be recorded during the entire monitoring period (up to 11 months old), because transmitter battery life was limited. Transmitters amplified signals 2000X (0.1–100 Hz). Signals were digitized (500 Hz, Digidata 1320A, Molecular Devices) and recorded (pCLAMP, Molecular Devices). Video images also were recorded, and mice were illuminated with red lighting so they would be visible when room lights were off. Electrophysiological recordings were evaluated (Clampex, Molecular Devices), and seizures were identified by high-frequency high-amplitude evolving rhythmic activity that lasted at least 10 s and exhibited an abrupt onset and offset. Corresponding video recordings were observed for seizure behaviors, defined as convulsive (≥ stage 3) or non-convulsive (< stage 3) (Racine, 1972).
2.3 Anatomy
After recordings were complete, mice were euthanized (>100 mg/kg pentobarbital, i.p.) and perfused through the ascending aorta at 15 ml/min for 2 min with 0.9% sodium chloride, 5 min with 0.37% sodium sulfide, 1 min with 0.9% sodium chloride, and 30 min with 4% formaldehyde in 0.1 M phosphate buffer (PB, pH 7.6). Brains were post-fixed overnight at 4°C. The right hippocampus was isolated, equilibrated in 30% sucrose in PB, frozen, and stored at −80°C. Later, hippocampi were thawed in 30% sucrose in PB, gently straightened, frozen, and sectioned transversely from the septal pole to the temporal pole with a microtome set at 40 µm. Sections were collected in 30% ethylene glycol and 25% glycerol in 50 mM PB and stored at −20°C.
Sections were rinsed in PB, mounted on gelatin-coated slides, and dried overnight. From each hippocampus, starting at a random point near the septal pole, a 1-in-12 series of sections was processed for Nissl staining with 0.25% thionin and then dehydrated and coverslipped with distyrene plasticizer xylene (DPX). A 1-in-24 series of adjacent sections was developed for Timm staining at room temperature in the dark for 45 min in 120 ml 50% gum arabic, 20 ml 2 M citrate buffer, 60 ml 0.5 M hydroquinone, and 1 ml 19% silver nitrate. After rinsing in water, sections were exposed to 5% sodium thiosulfate for 4 min before dehydration and coverslipping with DPX.
The optical fractionator method (West et al., 1991) was used to estimate the total number of Nissl-stained hilar neurons (large and small) per hippocampus. An average of 14 sections/mouse was analyzed. Borders of the hilus were outlined using a 10X objective. Sample sites were determined randomly and systematically with Stereo Investigator (MBF Bioscience). The counting grid was 125 × 125 µm, and the counting frame was 50 × 50 µm. Dissector height was total section thickness. Only nuclei not sectioned at the upper surface of the section were counted using a 100X objective. The average number of cells counted per mouse was 190. The coefficient of variation (0.191) was almost 2X larger than the mean coefficient of error (0.096), suggesting sufficient within-animal sampling.
Due to a technical problem, the Timm stain did not work in Experiment One. In Experiment Two, Timm-stained sections were evaluated for mossy fiber sprouting into the inner molecular layer of the dentate gyrus. An average of 7 sections/mouse was analyzed. Sections were assigned a score from 0 to 3 related to the quantity of supragranular, black Timm-staining, as described by Tauck and Nadler (1985). An average value was calculated for each mouse.
2.4 Positive-control groups
Positive control groups were used to validate methods and enable direct comparisons with phenotypes expected for temporal lobe epilepsy in mice. Pilocarpine-treated mice are a model of human temporal lobe epilepsy (Cavalheiro et al., 1996). Mice were treated with pilocarpine (300 mg/kg, i.p.) 20 min after atropine methylbromide (5 mg/kg, i.p.). Diazepam (10 mg/kg, i.p.) was administered 2 h after the onset of stage 3 or greater seizures, and repeated if needed to suppress convulsions. During recovery, mice were kept warm with a heating pad, and they received lactated Ringer’s with dextrose subcutaneously.
For Experiment One, 45 d old CD-1 mice of both sexes (Charles River) were treated with pilocarpine. Beginning one month after status epilepticus, positive-control mice were video-monitored for an average of at least 9 h/d for 27 d. For Experiment Two, 51 d old FVB mice of both sexes (Charles River) were treated with pilocarpine. One month later, they were implanted with a telemetric EEG transmitter. One week after that, continuous video-EEG monitoring began and continued for 40 ± 8 d (range, 21–61 d). After seizure monitoring was complete, positive-control mice were prepared for anatomical evaluation, as described above.
During data analysis investigators were blinded to whether mice had been treated with vehicle or DA. Statistical analyses were performed with SigmaPlot 12. Anatomical images were prepared with Adobe Photoshop 12. Only brightness and contrast of images were adjusted.
3. Results
3.1 Response of pregnant mice to DA
In Experiment One, one pregnant CD-1 mouse was treated with vehicle, and three were treated with 0.6 mg/kg DA. In Experiment Two, 10 pregnant FVB mice were treated with vehicle, and 15 were treated with 1.2 mg/kg DA. None of the vehicle-treated mice in Experiment One or Two displayed behavioral abnormalities during the 2 h period of observation following tail vein injection. Among the DA-treated mice, one of the three CD-1 mice and all of the 15 FVB mice displayed intense, repeated scratching, which is common after treatment with DA, even subconvulsive doses (Iverson et al., 1989; Tasker et al., 1991). Three DA-treated pregnant FVB mice exhibited convulsive seizures, during which two died. Offspring of the surviving mouse that experienced seizures were excluded from the study.
3.2. Experiment One
Over 43,000 mouse-hours of video recordings revealed no convulsive seizures in any of the CD-1 offspring that had been treated in utero with vehicle (n=10; 5 female, 5 male) or DA (n=30; 15 female, 15 male) (Figure 1A). In contrast, epilepsy developed in at least two of the five pilocarpine-treated CD-1 mice. One was observed to have one convulsive seizure (average, 0.03 seizures/d). The other was observed to have nine seizures (average, 0.28 seizures/d). The unit of variance in both Experiments One and Two was mouse, not litter.
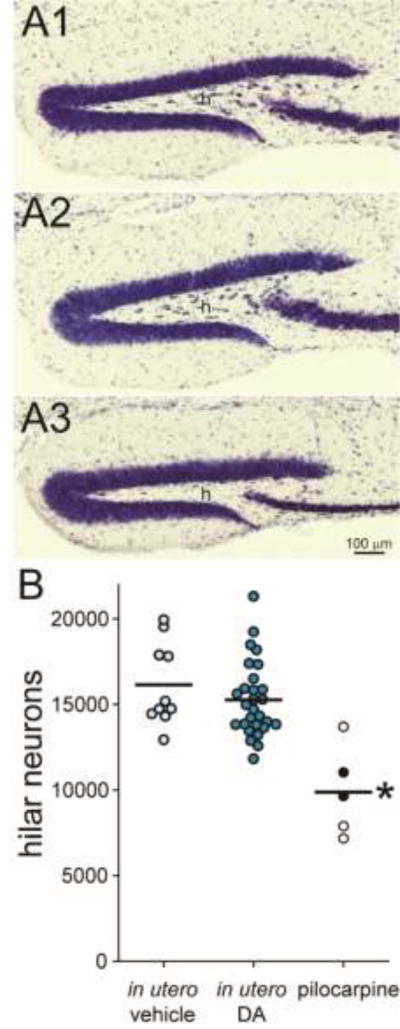
At 6 months old mice were perfused. There was no significant difference in the average number of hilar neurons in mice treated in utero with vehicle (16,100 ± 800) or DA (15,300 ± 400; p > 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s method) (Figure 2). Both vehicle- and DA-treated groups had more hilar neurons than the pilocarpine-treated group (9,900 ± 1200, p < 0.05).
Figure 2.
CD-1 mice exposed to domoic acid (DA) in utero failed to develop hilar neuron loss. Mice treated in utero with vehicle (A1) or DA (A2) display many neurons in the hilus (h). In contrast, a mouse treated with pilocarpine at 45 d old displays hilar neuron loss (A3). B The number of hilar neurons per hippocampus was similar in mice treated in utero with vehicle or DA but reduced in pilocarpine-treated mice (*p < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s method). Lines indicate group averages. Filled symbols indicate pilocarpine-treated mice that were observed to experience spontaneous convulsive seizures.
3.3 Experiment Two
To further test the hypothesis that in utero exposure to DA causes temporal lobe epilepsy, Experiment Two used a higher dose of DA, an earlier onset of seizure monitoring, continuous recording, a more sensitive seizure detection method, and a mouse strain and sex more susceptible to epilepsy. FVB mice have a low seizure threshold (Frankel et al., 2001), and some females develop epilepsy spontaneously (Mahler et al., 1996; Goelz et al., 1998; Rosenbaum et al., 2007; Silva-Fernandes et al., 2010).
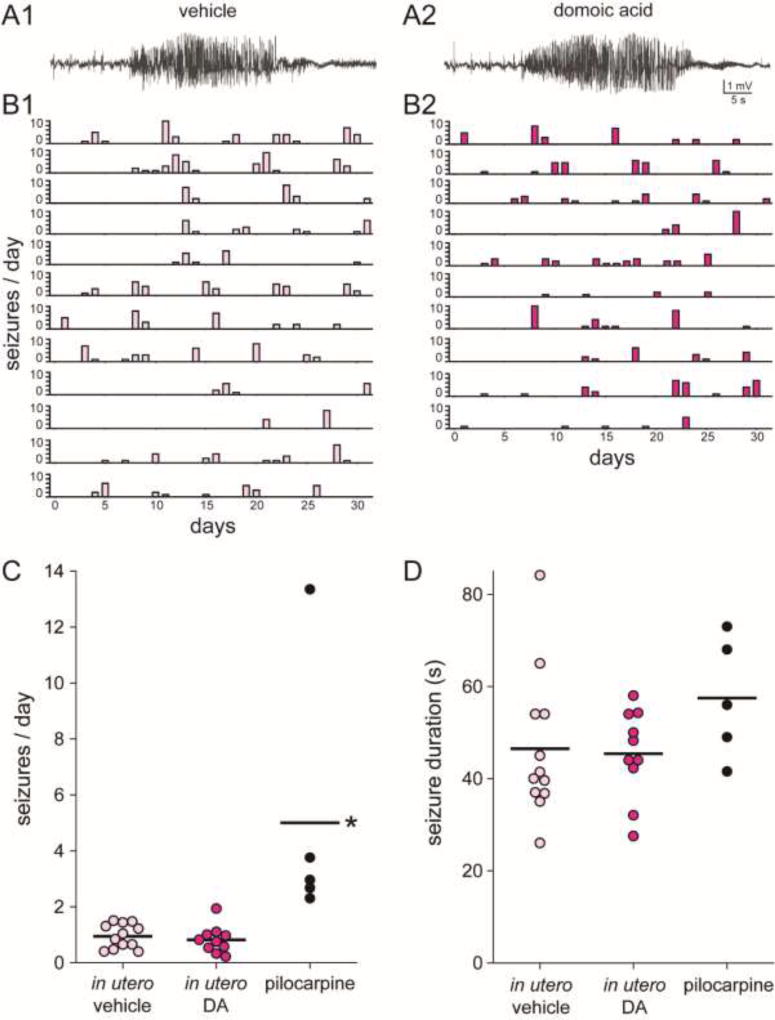
Continuous telemetric video-EEG recordings were obtained for one-month-long periods from 24 vehicle- and 26 DA-treated female FVB mice (Figure 1B). Seizures were not observed in any mice younger than 3 months old. Beginning after 3 months old, some mice in both groups displayed spontaneous recurrent seizures detected by hippocampal local field potential recording (Figure 3A). Seizures tended to occur in clusters (Figure 3B). A total of 12/24 vehicle- and 10/26 DA-treated mice developed epilepsy (p = 0.592, Chi-squared test) (Figure 1B). Average seizure frequency (0.95 and 0.82 seizures/day) (Figure 3C), seizure duration (46 and 45 s) (Figure 3D), and percentage of convulsive seizures (95 and 96%) were similar after in utero exposure to vehicle or DA, respectively (p > 0.5, t tests and Mann Whitney rank sum test).
Figure 3.
In utero exposure to domoic acid (DA) did not affect development of spontaneous epilepsy in female FVB mice. Seizures recorded from the hippocampus in 3 month old female FVB mice treated in utero with vehicle (A1) or DA (A2). Histograms of epileptic mice treated with vehicle (B1) or DA (B2), arranged from youngest (top) to oldest (bottom). C Seizure frequency was similar in female FVB mice that had been treated in utero with vehicle or DA but higher in pilocarpine-treated mice (*p < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s method). Lines indicate group averages. D Seizure duration was similar in female FVB mice that had been treated in utero with vehicle or DA and in pilocarpine-treated mice.
Results from spontaneously epileptic FVB mice were compared to those of a model of temporal lobe epilepsy. The proportion of pilocarpine-treated mice that developed epilepsy (5/5, 100%) was greater than that of female FVB mice over 3 months old that spontaneously developed epilepsy (22/38, 58%, p = 0.01, Fisher Exact test). Average seizure frequency in pilocarpine-treated mice (5.00 seizures/day) was over 5 times that of spontaneously epileptic female FVB mice (Figure 3C). However, average seizure duration (58 s) (Figure 3D) and percentage of convulsive seizures (86%) in pilocarpine-treated mice were not significantly different from that of spontaneously epileptic female FVB mice (p > 0.2, Kruskal-Wallis ANOVA on ranks).
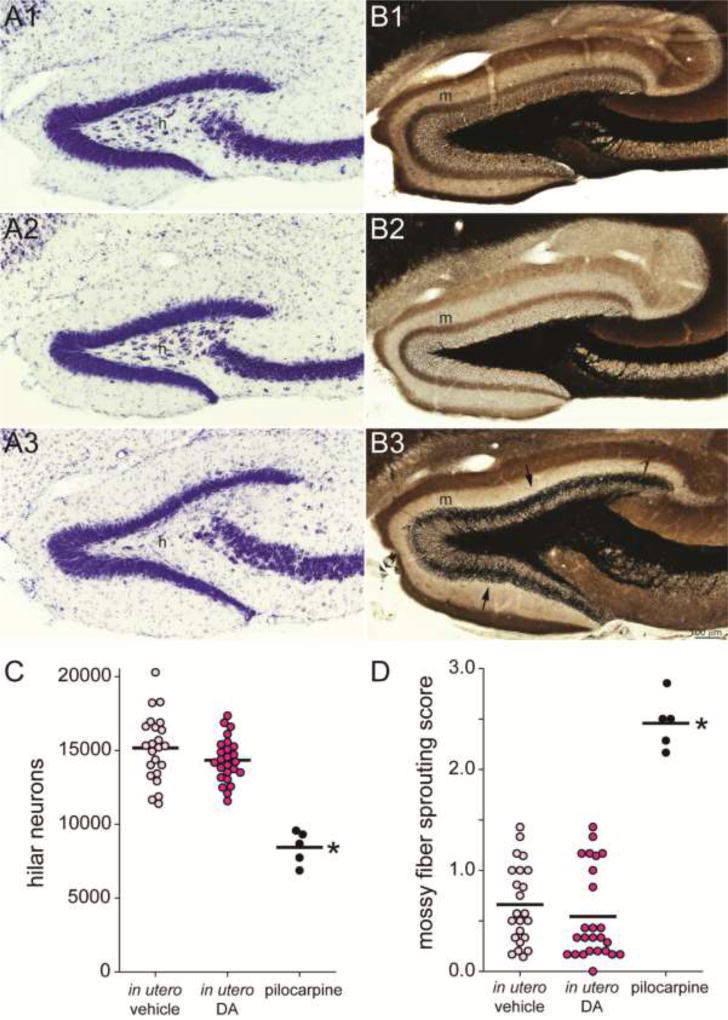
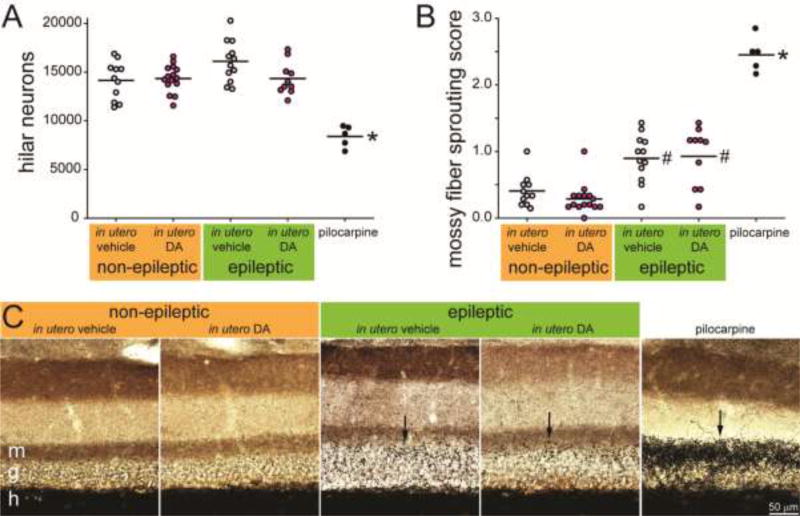
To test whether in utero exposure to DA causes hippocampal pathology typical of temporal lobe epilepsy, Nissl-stained hilar neuron numbers were estimated and mossy fiber sprouting was measured (Figure 4). Hilar neuron numbers were similar in mice treated in utero with vehicle (15,200 ± 500) or DA (14,300 ± 300; p = 0.123, ANOVA with Holm-Sidak method). Pilocarpine-treated FVB mice had significantly fewer hilar neurons (8400 ± 500; p < 0.001). Average mossy fiber sprouting scores were low and similar in mice treated in utero with vehicle (0.66 ± 0.08) or DA (0.54 ± 0.09; p > 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s method). Pilocarpine-treated FVB mice had a significantly higher average mossy fiber sprouting score (2.46 ± 0.12; p < 0.001).
Figure 4.
FVB mice exposed to domoic acid (DA) in utero failed to develop neuropathology of temporal lobe epilepsy. Mice treated in utero with vehicle (A1,B1) or DA (A2,B2) display many neurons in the hilus (h) and no dense mossy fiber sprouting into the molecular layer (m). In contrast, an FVB mouse treated with pilocarpine at 51 d old displays hilar neuron loss (A3) and dense mossy fiber sprouting (B3, arrows). C The number of hilar neurons per hippocampus was similar in mice treated in utero with vehicle or DA but reduced in epileptic pilocarpine-treated mice (*p < 0.001, ANOVA with Holm-Sidak method). Lines indicate group averages. D Average mossy fiber sprouting scores were similar in mice treated in utero with vehicle or DA but increased in epileptic pilocarpine-treated mice (*p < 0.05, Kruskal-Wallis ANOVA on ranks with Dunn’s method).
3.4 Spontaneous epilepsy in female FVB mice
Spontaneous epilepsy in female FVB mice has been reported previously (Mahler et al., 1996; Goelz et al., 1998; Rosenbaum et al., 2007; Silva-Fernandes et al., 2010) but has not been completely characterized. To test whether male FVB mice develop epilepsy spontaneously at a similar rate as females, eight male FVB mice (four treated in utero with vehicle and four with DA) were video-EEG monitored when they were 8–11 months old, an age when spontaneous epilepsy is common in female FVB mice (Figure 1). None of the male mice displayed spontaneous seizures. The proportion of female FVB mice over 3 months old that developed epilepsy (22/38, 58%) was significantly greater than that of males (0/8, p = 0.047, Fisher Exact test).
The cause of spontaneous epilepsy in female FVB mice is not known. From previous reports it remains unclear whether epilepsy was restricted to specific breeding pairs or more widespread. To begin investigating the inheritance of epilepsy in female FVB mice, their pedigrees were evaluated. Video-EEG epilepsy monitoring was conducted on 50 female mice that descended from 19 dams (10 vehicle- and 9 DA-treated). The daughters of 3 dams were evaluated when they were <3 months old, so it is unclear whether they would have developed epilepsy or not. For 7 dams, only one daughter >3 months old was evaluated; 3 were epileptic and 4 were not. For 3 dams, more than one daughter >3 months old was evaluated and all developed epilepsy (2/2, 2/2, and 3/3). The remaining 6 dams had more than one daughter >3 months old evaluated, but only some developed epilepsy (1/6, 1/3, 1/2, 3/5, 2/3, and 4/5).
To test whether spontaneously epileptic female FVB mice displayed differences in hippocampal anatomy compared to non-epileptic female FVB mice, hilar neuron numbers and mossy fiber sprouting were compared. There were no significant differences in the number of Nissl-stained hilar neurons (Figure 5A), but average mossy fiber sprouting scores were slightly higher in spontaneously epileptic FVB mice compared to non-epileptic FVB mice (Figure 5BC).
Figure 5.
Female FVB mice that developed epilepsy spontaneously did not develop classical neuropathology of temporal lobe epilepsy. A Non-epileptic and epileptic female FVB mice that had been exposed to vehicle or domoic acid (DA) in utero had similar numbers of hilar neurons per hippocampus, whereas epileptic pilocarpine-treated FVB mice displayed hilar neuron loss (*p < 0.001, ANOVA with Holm-Sidak method). Lines indicate group averages. B Average mossy fiber sprouting scores were similar and low in non-epileptic female FVB mice treated in utero with vehicle or DA, slightly higher in FVB mice that developed epilepsy spontaneously (#p ≤ 0.002), and highest in epileptic pilocarpine-treated mice (*p < 0.001). C Female FVB mice treated in utero with vehicle or DA that developed epilepsy spontaneously display mild mossy fiber sprouting into the inner molecular layer (m) but not dense mossy fiber sprouting seen in an epileptic pilocarpine-treated mouse. Images obtained from the superior blade of the granule cell layer (g) in sections at the mid-septotemporal axis of the hippocampus. h = hilus. Arrows indicate mossy fiber sprouting.
4. Discussion
4.1 Summary
Dakshinamurti et al. (1993) reported that exposure of mice to a single subconvulsive dose of DA at E13 caused epileptiform EEG activity and progressively severe excitotoxic hippocampal neuron damage, especially in the dentate gyrus. That report led to the hypothesis that in utero exposure to low-dose DA causes temporal lobe epilepsy to develop later in life (Stewart, 2010). We replicated the methods of Dakshinamurti et al. (1993) and extended the analysis with long-term seizure monitoring, a higher dose of DA, and a mouse strain more susceptible to epilepsy. The main findings of the present study are that exposing mice to DA in utero did not make them more likely to develop spontaneous seizures, hilar neuron loss, or mossy fiber sprouting. These findings raise doubts about the hypothesis that in utero exposure to low-dose DA causes temporal lobe epilepsy in humans.
4.2 In utero low-dose exposure to DA did not cause spontaneous recurrent seizures
Dakshinamurti et al. (1993) reported that after exposure to 0.6 mg/kg DA at E13 epileptiform EEG activity became progressively more severe, but no clinical seizures were observed in mice up to 30 d old. They concluded that longer observations were needed to determine whether clinical seizures developed. Experiment One of the present study replicated their treatment protocol and then video-monitored mice for convulsive seizures 9 h/d every day from 2–6 months of age. No convulsive seizures were observed. Experiment Two used a higher dose of DA, a mouse strain and sex more susceptible to epilepsy, and continuous video-EEG monitoring for 1-month-long periods in mice at 1–11 months of age. There were no significant differences between vehicle- and DA-treated mice. Together, these findings suggest that exposure to subconvulsive doses of DA at E13 does not cause epilepsy in mice.
4.3 In utero low-dose exposure to DA did not cause neuropathology of temporal lobe epilepsy
Dakshinamurti et al. (1993) reported that after exposure to 0.6 mg/kg DA at E13, mice developed densely stained, swollen, and distorted neurons with pyknotic appearance in CA3 and the dentate gyrus. These neuropathological changes were evident at 14 d old and were more severe at 30 d, the latest age examined. In the present study, the number of hilar neurons per hippocampus was estimated. Hilar neurons are even more vulnerable than granule cells and CA3 pyramidal neurons in mouse models of temporal lobe epilepsy (Buckmaster et al., 2017) and in human patients with temporal lobe epilepsy (Margerison and Corsellis, 1966). There was no significant loss of hilar neurons in mice exposed to 0.6 or 1.2 mg/kg DA at E13. In addition, Nissl staining revealed no obvious abnormalities in granule cells or pyramidal neurons in mice exposed to DA in utero. However, we did not quantify neuron numbers in CA1, CA3, or the granule cell layer.
To further test whether neuropathology develops in mice exposed to DA in utero we measured mossy fiber sprouting, which is a classic abnormality found in patients with temporal lobe epilepsy (Sutula et al., 1989; de Lanerolle et al., 1989; Houser et al., 1990; Babb et al., 1991). Positive-control pilocarpine-treated mice developed high levels of aberrant mossy fiber sprouting, evident as a dense black band in the inner molecular layer of the dentate gyrus. Mice exposed to 1.2 mg/kg DA in utero did not. Together with results from hilar neuron analyses, these findings suggest that exposure to subconvulsive doses of DA at E13 does not cause neuropathology of temporal lobe epilepsy in mice.
4.4 Caveats
One might question whether the dose of DA used was too low to cause temporal lobe epilepsy. Dakshinamurti et al. (1993) chose a dose that they reported to be one-fourth the convulsive dose. That low dose is consistent with the possibility of unrecognized dietary exposure to DA in pregnant women. Experiment One of the present study replicated their treatment protocol (0.6 mg/kg). Experiment Two used a higher dose of DA (1.2 mg/kg). Minutes after intravenous injection of 1.2 mg/kg DA, all mice displayed intense scratching and in some cases, seizures and death. Therefore, doses used in the present study were likely to be sufficient or exceeding those that might go undetected by pregnant women.
Results of the present study do not eliminate the possibility that in utero exposure to subconvulsive doses at different developmental stages or for longer periods (as may be true in sea lions) might cause temporal lobe epilepsy in humans. The rationale for the timing of in utero treatment by Dakshinamurti et al. (1993) was the coincident peak in hippocampal neurogenesis (Angevine, 1965), by which time neurons in rat depolarize to kainate (LoTurco et al., 1995). After intravenous administration, DA accumulates in amniotic fluid and remains in the fetal brain without evidence of elimination for at least 24 h (Maucher Fuquay et al., 2012). Therefore, the protocol used probably caused prolonged brain exposure at a potentially sensitive developmental stage. Nevertheless, in utero exposure to DA did not cause epilepsy to develop later in life, and studies have not found repeated doses to enhance neurotoxicity over a single dose (Costa et al., 2010). However, there is evidence that humans are more sensitive to DA than mice (Lefebvre and Robertson, 2010).
Results of the present study do not support the development of epilepsy (spontaneous recurrent seizures) after prenatal low-dose exposure to DA in mice. However, these findings do not preclude or refute long-term effects of DA after in utero exposure (see Introduction). Low doses of DA postnatally in young rats have been reported to cause hippocampal synaptic reorganization and neuron loss (Doucette et al., 2004; Bernard et al., 2007), including loss of specific subtypes of inhibitory interneurons (Gill et al., 2010b), behavioral abnormalities (Doucette et al., 2003, 2007), reduced seizure threshold (Gill et al., 2010a), and seizure-like behaviors (Doucette et al., 2004). However, other studies found little evidence for changes in brain morphology (Xi et al., 1997) and only subtle changes in behavior (Levin et al., 2006). Dakshinamurti et al. (1993) reported abnormal EEG and reduced seizure threshold after low-dose in utero exposure to DA, but not spontaneous seizures. Similarly, we found no spontaneous seizures in CD-1 mice after the same exposure, but did not test seizure threshold. Local field potential recordings from the hippocampus in vehicle- and DA-treated FVB mice were not obviously different, but detailed quantitative analyses were not performed.
4.5 Characterization of spontaneous epilepsy in female FVB mice
Seizures began in female FVB mice after 3 months old, confirming previous reports (Mahler et al., 1996; Goelz et al., 1998; Rosenbaum and VandeWoude, 2007; Silva-Fernandes et al., 2010). In addition, the present study revealed that epilepsy developed spontaneously in many litters and in over half of female FVB mice. Seizures tended to cluster, occurred approximately 30 times per month, and lasted approximately 45 s. They were generalized tonic-clonic convulsions 95% of the time, and could be recorded in the hippocampus. Recordings were obtained only from the hippocampus, so it remains unclear where seizures began. In mice that spontaneously developed epilepsy, mild mossy fiber sprouting occurred, similar to that after a limited number of provoked seizures during kindling protocols (Sutula et al., 1988). The cause/effect relationship of mossy fiber sprouting and epilepsy is controversial (Buckmaster, 2014). Other investigators now can consider these characteristics when deciding whether to use female FVB mice.
Highlights.
Low-dose in utero exposure to DA has been proposed to cause temporal lobe epilepsy
We replicated and extended a previous mouse study
A subconvulsive dose of DA at mid-gestation did not cause epilepsy to develop in mice
A subconvulsive dose of DA at mid-gestation did not cause TLE neuropathology in mice
Spontaneous epilepsy in female FVB mice is characterized
Acknowledgments
Supported by the National Science Foundation (grant number ES021960) and the National Institutes of Health (grant numbers ES021960 and OD010989).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries. 2002;25:704–726. [Google Scholar]
- Angevine JB., Jr Time of neuron origin in the hippocampal region. An autoradiographic study in the mouse. Exp Neurol Suppl. 1965;2:1–70. [PubMed] [Google Scholar]
- Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. J Neurosci. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejarano AC, VanDola FM, Gulland FM, Rowles TK, Schwacke LH. Production and toxicity of the marine biotoxin domoic acid and its effects on wildlife: a review. Hum Ecolog Risk Assess. 2008;14:544–567. [Google Scholar]
- Bernard PB, MacDonald DS, Gill DA, Ryan CL, Tasker RA. Hippocampal mossy fiber sprouting and elevated trkB receptor expression following systemic administration of low dose domoic acid during neonatal development. Hippocampus. 2007;17:1121–1133. doi: 10.1002/hipo.20342. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS. Does mossy fiber sprouting give rise to the epileptic state? Adv Exp Med Biol. 2014;813:161–168. doi: 10.1007/978-94-017-8914-1_13. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Abrams E, Wen X. Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy. J Comp Neurol. 2017;525:2592–2610. doi: 10.1002/cne.24226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Lew FH. Rapamycin suppresses mossy fiber sprouting but not seizure frequency in a mouse model of temporal lobe epilepsy. J Neurosci. 2011;31:2337–2347. doi: 10.1523/JNEUROSCI.4852-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster PS, Wen X, Toyoda I, Gulland FM, Van Bonn W. Hippocampal neuropathology of domoic acid-induced epilepsy in California sea lions (Zalophus californianus) J Comp Neurol. 2014;522:1691–1706. doi: 10.1002/cne.23509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalheiro EA, Santos NF, Priel MR. The pilocarpine model of epilepsy in mice. Epilepsia. 1996;37:1015–1019. doi: 10.1111/j.1528-1157.1996.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor–mediated excitotoxicity in humans. Ann Neurol. 1995;37:123–126. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- Colman JR, Nowocin KJ, Switzer RC, Trusk TC, Ramsdell JS. Mapping and reconstruction of domoic acid-induced neurodegeneration in the mouse brain. Neurotoxicol Teratol. 2005;27:753–767. doi: 10.1016/j.ntt.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Faustman EM. Domoic acid as a developmental neurotoxin. Neurotoxicology. 2010;31:409–423. doi: 10.1016/j.neuro.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J Neurosci. 1993;13:4486–4495. doi: 10.1523/JNEUROSCI.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lanerolle NC, Kim JH, Robbins RJ, Spencer DD. Hippocampal interneuron loss and plasticity in human temporal lobe epilepsy. Brain Res. 1989;495:387–395. doi: 10.1016/0006-8993(89)90234-5. [DOI] [PubMed] [Google Scholar]
- Doucette TA, Bernard PB, Husum H, Perry MA, Ryan CL, Tasker RAR. Low doses of domoic acid during postnatal development produce permanent changes in rat behaviour and hippocampal morphology. Neurotox Res. 2004;6:555–563. doi: 10.1007/BF03033451. [DOI] [PubMed] [Google Scholar]
- Doucette TA, Bernard PB, Tasker RAR, Ryan CL. Low doses of non-NMDA glutamate receptor agonists alter neurobehavioural development in the rat. Neurotox Teratol. 2003;25:473–479. doi: 10.1016/s0892-0362(03)00034-5. [DOI] [PubMed] [Google Scholar]
- Doucette TA, Ryan CL, Tasker RAR. Gender-based changes in cognition and emotionality in a new rat model of epilepsy. Amino Acids. 2007;32:317–322. doi: 10.1007/s00726-006-0418-7. [DOI] [PubMed] [Google Scholar]
- Engel J, Jr, Williamson PD, Wieser HG. Mesial temporal lobe epilepsy. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven; 1997. pp. 2417–2426. [Google Scholar]
- Erdner DL, Dyble J, Parsons ML, Stevens RC, Hubbard KA, Wrabel ML, Moore SK, Lefebvre KA, Anderson DM, Bienfang P, Bidigare RR, Parker MS, Moeller P, Brand LE, Trainer VL. Centers for oceans and human health: a unified approach to the challenge of harmful algal blooms. Env Health. 2008;7(suppl 2):52. doi: 10.1186/1476-069X-7-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel WN, Taylor L, Beyer B, Tempel BL, White HS. Electroconvulsive thresholds of inbred mouse strains. Genomics. 2001;74:306–312. doi: 10.1006/geno.2001.6564. [DOI] [PubMed] [Google Scholar]
- French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993;34:774–780. doi: 10.1002/ana.410340604. [DOI] [PubMed] [Google Scholar]
- Garrison DL, Conrad SM, Eilers PP, Waldron EM. Confirmation of domoic acid production by Pseudonitzschia australis (Bacillariophyceae) cultures. J Phycol. 1992;28:604–607. [Google Scholar]
- Gill DA, Bastlund JF, Watson WP, Ryan CL, Reynolds DS, Tasker RA. Neonatal exposure to low-dose domoic acid lowers seizure threshold in adult rats. Neuroscience. 2010a;169:1789–1799. doi: 10.1016/j.neuroscience.2010.06.045. [DOI] [PubMed] [Google Scholar]
- Gill DA, Ramsay SL, Tasker RA. Selective reductions in subpopulations of GABAergic neurons in a developmental rat model of epilepsy. Brain Res. 2010b;1331:114–123. doi: 10.1016/j.brainres.2010.03.054. [DOI] [PubMed] [Google Scholar]
- Goelz MF, Mahler J, Harry J, Myers P, Clark J, Thigpen JE, Forsythe DB. Neuropathologic findings associated with seizures in FVB mice. Lab Anim Sci. 1998;48:34–37. [PubMed] [Google Scholar]
- Goldstein T, Mazet JAK, Zabka T, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc R Soc B Biol Sci. 2008;275:267–276. doi: 10.1098/rspb.2007.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KS, Burbacher TM, Faustman EM, Gratttan L. Domoic acid: neurobehavioral consequences of exposure to a prevalent marine biotoxin. Neurotoxicol Teratol. 2010;32:132–141. doi: 10.1016/j.ntt.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Logroscino G, Benn EK, Katri N, Cascino G, Hauser WA. Estimating risk for developing epilepsy. Neurology. 2011;76:23–27. doi: 10.1212/WNL.0b013e318204a36a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Accumulation of abnormal adult generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiolski EM, Kendrick PS, Frame ER, Myers MS, Bammler TK, Beyer RP, Farin FM, Wilkerson HW, Smith DR, Marcinek DJ, Lefebvre KA. Chronic low-level domoic acid exposure alters gene transcription and impairs mitochondrial function in the CNS. Aquat Toxicol. 2014;155:151–159. doi: 10.1016/j.aquatox.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houser CR, Miyashiro JE, Swartz BE, Walsh GO, Rich JR, Delgado-Escueta AV. Altered patterns of dynorphin immunoreactivity suggest mossy fiber reorganization in human hippocampal epilepsy. J Neurosci. 1990;10:267–282. doi: 10.1523/JNEUROSCI.10-01-00267.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson F, Truelove J. Toxicology and seafood toxins: domoic acid. Nat Toxins. 1994;2:334–339. doi: 10.1002/nt.2620020514. [DOI] [PubMed] [Google Scholar]
- Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok E. Domoic acid poisoning and mussel-associated intoxication: preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem Toxicol. 1989;27:377–384. doi: 10.1016/0278-6915(89)90143-9. [DOI] [PubMed] [Google Scholar]
- Kitz S, Thalhammer JG, Glantschnigg U, Wrzosek M, Klang A, Halasz P, Shouse MN, Pakozdy A. Feline temporal lobe epilepsy: review of the experimental literature. J Vet Intern Med. 2017;31:633–640. doi: 10.1111/jvim.14699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre KA, Robertson A. Domoic acid and human exposure risks: a review. Toxicon. 2010;56:218–230. doi: 10.1016/j.toxicon.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Levin ED, Pang WG, Harrison J, Williams P, Petro A, Ramsdell JS. Persistent neurobehavioral effects of early postnatal domoic acid exposure in rats. Neurotox Teratol. 2006;28:673–680. doi: 10.1016/j.ntt.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Levin ED, Pizarro K, Pang WG, Harrison J, Ramsdell JS. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicol Teratol. 2005;27:719–725. doi: 10.1016/j.ntt.2005.06.017. [DOI] [PubMed] [Google Scholar]
- LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287–1298. doi: 10.1016/0896-6273(95)90008-x. [DOI] [PubMed] [Google Scholar]
- Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice. Toxicol Pathol. 1996;24:710–716. doi: 10.1177/019262339602400606. [DOI] [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. Brain. 1966;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Mathern GW, Babb TL, Vickrey BG, Melendez M, Pretorius JK. The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain. 1995;118:105–118. doi: 10.1093/brain/118.1.105. [DOI] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: Video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol. 2012;238:156–167. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Maucher Fuquay J, Muha N, Wang Z, Ramsdell JS. Toxicokinetics of domoic acid in the fetal rat. Toxicology. 2012;294:36–41. doi: 10.1016/j.tox.2012.01.012. [DOI] [PubMed] [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, Gulland FM, Thomson RE, Cochlan WP, Trainer VL. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys Res Lett. 2016;43:10366–10376. doi: 10.1002/2016GL070023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, Brierley JB. Prolonged epileptic seizures in primates. Ischemic cell change and its relation to ictal physiological events. Arch Neurol. 1973;28:10–17. doi: 10.1001/archneur.1973.00490190028002. [DOI] [PubMed] [Google Scholar]
- Mills BD, Pearce HL, Khan O, Jarrett BR, Fair DA, Lahvis GP. Prenatal domoic acid exposure disrupts mouse pro-social behavior and functional connectivity MRI. Behav Brain Res. 2016;308:14–23. doi: 10.1016/j.bbr.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muha N, Ramsdell JS. Domoic acid induced seizures progress to a chronic state of epilepsy in rats. Toxicon. 2011;57:168–171. doi: 10.1016/j.toxicon.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Nadler JV, Perry BW, Cotman CW. Selective reinnervation of hippocampal area CA1 and the fascia dentata after destruction of CA3-CA4 afferents with kainic acid. Brain Res. 1980;182:1–9. doi: 10.1016/0006-8993(80)90825-2. [DOI] [PubMed] [Google Scholar]
- Pérez-Gómez A, Tasker RA. Domoic acid as a neurotoxin. In: Kostrzewa RM, editor. Handbook of Neurotoxicity. Springer; New York: 2014. pp. 399–419. [Google Scholar]
- Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990;322:1775–1780. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- Potera C. Marine toxin hinders cognitive development. Env Health Perspect. 2006;114:A94. doi: 10.1289/ehp.114-a94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesney LF. Clinical and EEG features of complex partial seizures of temporal lobe origin. Epilepsia. 1986;27(Suppl 2):S27–S45. doi: 10.1111/j.1528-1157.1986.tb05738.x. [DOI] [PubMed] [Google Scholar]
- Ramsdell JS, Gulland FM. Domoic acid epileptic disease. Mar Drugs. 2014;12:1185–207. doi: 10.3390/md12031185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell JS, Zabka TS. In utero domoic acid toxicity: a fetal basis to adult disease in the California sea lion (Zalophus californianus) Mar Drugs. 2008;6:262–290. doi: 10.3390/md20080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Rosenbaum MD, VandeWoude S, Bielefeldt-Ohmann H. Sudden onset of mortality within a colony of FVB/n mice. Lab Animal. 2007;36:15–16. doi: 10.1038/laban0607-15. [DOI] [PubMed] [Google Scholar]
- Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles T, Silvagni P, Silver M, Spraker T, Trainer V, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–844. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- Shiotani M, Cole TB, Hong S, Park JJY, Griffith WC, Burbacher TM, Workman T, Costa LG, Faustman EM. Neurobehavioral assessment of mice following repeated oral exposures to domoic acid during prenatal development. Neurotoxicol Teratol. 2017;64:8–19. doi: 10.1016/j.ntt.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Silva-Fernandes A, Oliveira P, Sousa N, Maciel P. Motor and behavioural abnormalities associated with persistent spontaneous epilepsy in the fvb/n mouse strain. Scand J Lab Anim Sci. 2010;37:213–222. [Google Scholar]
- Stewart GR, Zorumski CF, Price MT, Olney JW. Domoic acid: a dementia-inducing excitotoxic food poison with kainic acid receptor specificity. Exp Neurol. 1990;110:127–138. doi: 10.1016/0014-4886(90)90057-y. [DOI] [PubMed] [Google Scholar]
- Stewart I. Environmental risk factors for temporal lobe epilepsy – Is prenatal exposure to the marine algal neurotoxin domoic acid a potentially preventable cause? Med Hypotheses. 2010;74:466–481. doi: 10.1016/j.mehy.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Sutula T, Cascino G, Cavazos J, Parada I, Ramirez L. Mossy fiber synaptic reorganization in the epileptic human temporal lobe. Ann Neurol. 1989;26:321–330. doi: 10.1002/ana.410260303. [DOI] [PubMed] [Google Scholar]
- Sutula T, He XX, Cavazos J, Scott G. Synaptic reorganization in the hippocampus induced by abnormal functional activity. Science. 1988;239:1147–1150. doi: 10.1126/science.2449733. [DOI] [PubMed] [Google Scholar]
- Tanemura K, Igarashi K, Matsugami TR, Aisaki K, Kitajima S, Kanno J. Intrauterine environment-genome interaction and children’s development (2): Brain structure impairment and behavioral disturbance induced in male mice offspring by a single intraperitoneal administration of domoic acid (DA) to their dams. J Toxicol Sci. 2009;34(Special Issue II):SP279–SP286. doi: 10.2131/jts.34.sp279. [DOI] [PubMed] [Google Scholar]
- Tasker RA. Domoic acid and other amnesic toxins: toxicological profile. In: Gopalakrishnakone P, Haddad V Jr, Tubaro A, Kim E, Kem WR, editors. Marine and Freshwater Toxins. Springer; Dordrecht: 2016. pp. 93–112. [Google Scholar]
- Tasker RAR, Connell BJ, Strain SM. Pharmacology of systemically administered domoic acid in mice. Can J Physiol Pharmacol. 1991;69:378–382. doi: 10.1139/y91-057. [DOI] [PubMed] [Google Scholar]
- Tauck DL, Nadler JV. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985;5:1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurological sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Eng J Med. 1990;322:1781–1787. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS. Embryonic exposure to domoic Acid increases the susceptibility of zebrafish larvae to the chemical convulsant pentylenetetrazole. Environ Health Perspect. 2007;115:1547–1552. doi: 10.1289/ehp.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci. 2013;33:11100–11115. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells ML, Trainer VL, Smayda TJ, Karlson BS, Trick CG, Kudela RM, Ishikawa A, Bernard S, Wulff A, Anderson DM, Cochlan WP. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae. 2015;49:68–93. doi: 10.1016/j.hal.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wright JLC, Boyd RK, de Freitas A, Falk M, Foxall RA, Jamieson WD, Laycock MV, McCulloch AW, McInnes AG, Odense P, Pathak VP, Quilliam MA, Ragan MA, Sim PG, Thibault P, Walter JA. Identification of domoic acid, a neuroexcitatory amino acid, in toxic mussels from eastern Prince Edward Island. Can J Chem. 1989;67:481–490. [Google Scholar]
- Xi D, Peng YG, Ramsdell JS. Domoic acid is a potent neurotoxin to neonatal rats. Nat Toxins. 1997;5:74–79. doi: 10.1002/(SICI)(1997)5:2<74::AID-NT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Zaczek R, Coyle JT. Excitatory amino acid analogues: neurotoxicity and seizures. Neuropharmacology. 1982;21:15–26. doi: 10.1016/0028-3908(82)90205-2. [DOI] [PubMed] [Google Scholar]
- Zuloaga DG, Lahvis GP, Mills B, Pearce HL, Turner J, Raber J. Fetal domoic acid exposure affects lateral amygdala neurons, diminishes social investigation and alters sensory-motor gating. NeuroToxicology. 2016;53:132–140. doi: 10.1016/j.neuro.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]