Figure 1. c-Myb represses Bmf and Bim expression in pro-B cells.
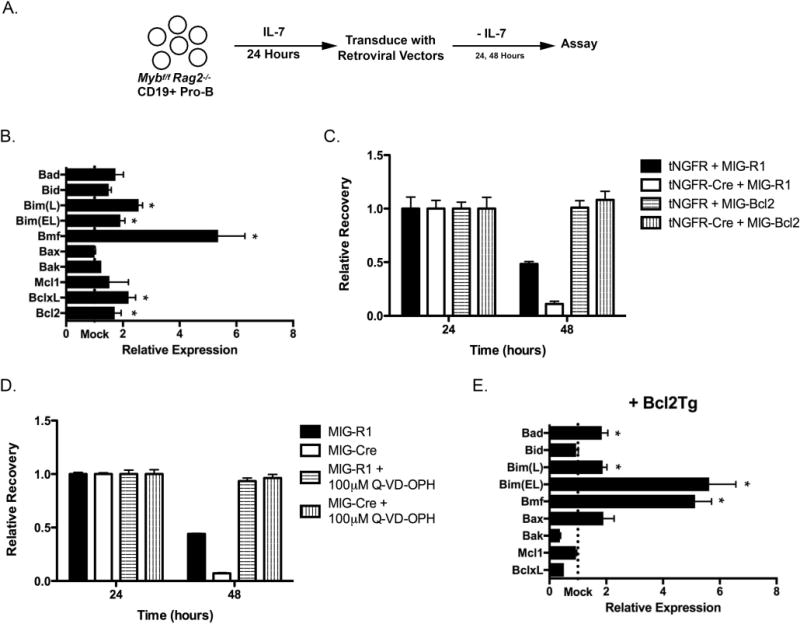
(A) The experimental system to analyze the role of c-Myb during the pro-B cell stage. Pro-B cells from Mybf/f Rag2−/− mice were positively selected using anti-CD19 coated magnetic beads and cultured for 24 hours in the presence of IL-7. These cells were subsequently transduced with retroviruses that produce a bicistronic message that encodes the gene of interest followed by an internal ribosome entry site (IRES) and a reporter gene, either GFP (MIG-R1) or a truncated human nerve growth factor receptor (tNGFR). Following retrovirus transduction, pro-B cells were placed in culture in the absence of exogenous IL-7 to measure the intrinsic survival of these cells. Every 24 hours, cells were analyzed for tNGFR and/or GFP expression as well as total cells per well. The number of tNGFR+ GFP+ cells per well at 24 hours post-transduction was set as 1. The relative recovery of tNGFR+ GFP+ cells at subsequent time points was determined as a ratio compared to the total number of tNGFR+ GFP+ cells present at 24 hours. (B) Mybf/f Rag2−/− CD19+ pro-B cells were transduced with MIG-R1 or MIG-Cre, cultured for 24 hours in the absence of IL-7 and electronically sorted based on GFP expression. Total cellular RNA was harvested and specific mRNA expression was analyzed by quantitative RT-PCR. Gene expression was normalized to the expression of Hprt. “Mock” represents the expression of each gene in MIG-R1-transduced cells. n=4 *, p < 0.05 (C) Mybf/f Rag2−/− CD19+ pro-B cells were cotransduced with tNGFR or tNGFR-Cre and MIG-R1 or MIG-Bcl2 and cultured in the absence of IL-7. Cells were analyzed 24 and 48 hours post-transduction by flow cytometry and relative recovery was determined. Retrovirus transductions were done in triplicate. Retrovirus transductions were done in triplicate. Data are representative of 3 independent experiments. (D) Mybf/f Rag2−/− CD19+ pro-B cells were transduced with MIG-R1 or MIG-Cre and cultured in the absence of IL-7 and the presence of 100 μM Q-VD-OPH. Cells were analyzed 24 and 48 hours post-transduction by flow cytometry and relative recovery was determined. Data are representative of 2 independent experiments. (E) Mybf/f Rag2−/− Bcl2Tg CD19+ pro-B cells were transduced with MIG-R1 or MIG-Cre, cultured for 48 hours in the absence of IL-7 and electronically sorted based on GFP expression. Total cellular RNA was harvested and analyzed by quantitative RT-PCR. Gene expression was normalized the expression of Hprt. “Mock” represents the expression of each gene in MIG-R1-transduced cells. n=4 *, p < 0.05
