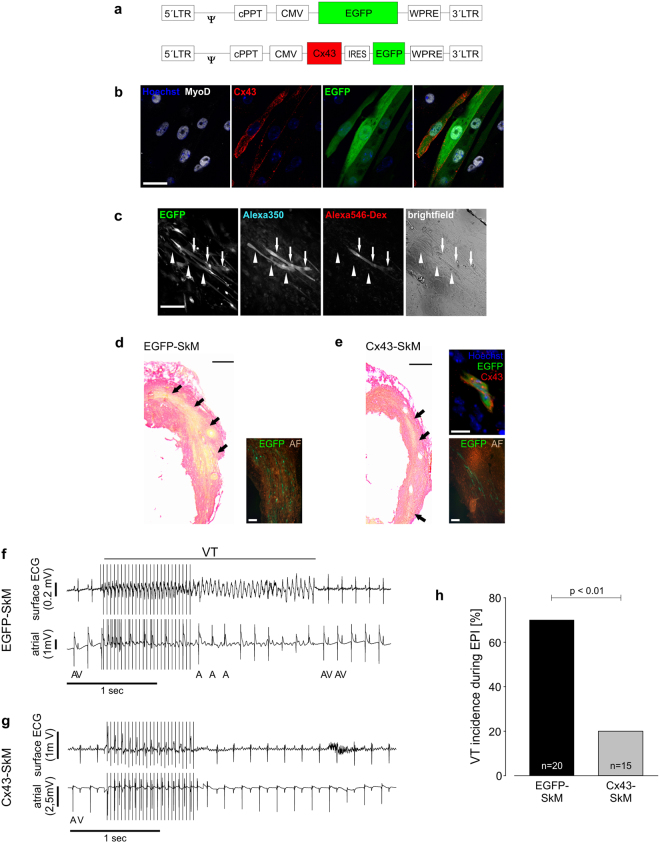
Figure 1.
Lentivirus-mediated Cx43 transduction of SkM results in functional gap junction formation in vitro and in vivo. (a) Scheme of the control EGFP and the bicistronic Cx43 lentiviral vectors. (b) Immunostainings of cultured, lentivirus transduced EGFP+ (green, third panel from left; nuclear Hoechst stain, blue) SkM prove expression of MyoD (white, left panel) and membrane-located Cx43 (red, second panel from left); the right panel is an overlay of all three pictures. (c) In vitro dye transfer in differentiated transgenic myotubes (EGFP+ , left); patch loading of the upper myotube (arrows) results in progressive dye transfer of Alexa 350 (middle left), but not of Alexa 546-dextran (middle right) into the neighbouring EGFP+ SkM (arrowheads). A brightfield image (right) shows a dense monolayer of differentiated and elongated myocytes. (d,e) Sirius Red staining of infarcted hearts 12–14 days after the lesion reveals engraftment of EGFP (d) and Cx43-EGFP (e) ex vivo transduced SkM (fibrotic tissue red, viable cells yellow) in the transmural scar area. Macroscopic imaging and quantitative morphometry revealed in average 19.185 ± 18.743 and 5.338 ± 4.552 engrafted cells in EGFP-SkM and Cx43-SkM engrafted hearts, respectively (n = 5 each). Insets show EGFP+ SkM (green, autofluorescence brown) or Cx43 immunostaining (red; nuclear Hoechst stain, blue), of engrafted Cx43-SkM (e, upper right). (f) Burst stimulation induces self-terminating VT in a representative EGFP-SkM transplanted mouse in vivo. (g) No VT is evoked in a representative Cx43-SkM transplanted mouse upon burst stimulation. (f,g) Top trace, surface ECG; bottom trace, atrial intracardiac lead; A, atrium; V, ventricle. (h) Statistics of VT incidence upon burst stimulation in vivo reveals prominent reduction of VT inducibility after engraftment of Cx43-SkM compared to EGFP-SkM (p < 0.01). Scale bars: b = 30 µm; c = 200 µm; d,e = 500 µm; d,e insets = 100 µm; e upper right inset = 10 µm.