Abstract
This study explored the effects of butylated hydroxytoluene (BHT) and ethoxyquin (EQ) and ethyl ether extracts, ethyl acetate extracts (EAE), acetone extracts, ethanol extracts and aqueous extracts of Ginkgo biloba leaves (EGbs) on lipid oxidation in a linoleic acid emulsion, fish flesh and fish feed and in hydroxyl radical (·OH)-treated carp erythrocytes. The linoleic acid, fish flesh and fish feed were incubated with BHT, EQ and EGbs at 45°C for 8 d, respectively, except for the control group. The lipid oxidation in the linoleic acid emulsion, fish flesh and fish feed was then measured by the ferric thiocyanate method or thiobarbituric acid method. The carp erythrocytes were treated with BHT, EQ or EGbs in the presence of 40 μmol/L FeSO4 and 20 μmol/L H2O2 at 37°C for 6 h, except for the control group. Oxidative stress and apoptosis parameters in carp erythrocytes were then evaluated by the commercial kit. The results showed that BHT, EQ and EGbs inhibited lipid oxidation in the linoleic acid emulsion, fish flesh and fish feed and ·OH-induced phosphatidylserine exposure and DNA fragmentation (the biomarkers of apoptosis) in carp erythrocytes. Furthermore, BHT, EQ and EGbs decreased the generation of reactive oxygen species (ROS), inhibited the oxidation of cellular components and restored the activities of enzymatic antioxidants in ·OH-treated carp erythrocytes. Of all examined EGbs, EAE showed the strongest effects. The effects of EAE on lipid oxidation in the linoleic acid emulsion and on superoxide anion and malonaldehyde levels, catalase activity and apoptosis in ·OH-treated carp erythrocytes were equivalent to or stronger than those of BHT. Moreover, these results indicated that the inhibition order of EGbs on the generation of ROS and oxidation of cellular components in fish erythrocytes approximately agreed with that for the food and feed materials tested above. And, the antioxidative and anti-apoptotic effects of EGbs were positively correlated with their flavonoid content. Taken together, these results revealed that the fish erythrocyte system can be used as an experimental model to evaluate lipid oxidation in food and feed ingredients. The EAE can be used as a potential natural antioxidant or apoptosis inhibitor. The inhibition effects of EGbs on lipid oxidation and apoptosis may be due to the presence of flavonoid compounds.
Keywords: Ginkgo biloba, Lipid oxidation, Apoptosis, Fish erythrocyte, Flavonoids
1. Introduction
Lipid oxidation is a common problem in foods and feeds (Fritsche and Johnston, 1988, Wqsowicz et al., 2004). Lipids can undergo peroxidation in a chain reaction with reactive oxygen species (ROS), including the superoxide anion (), hydrogen peroxide (H2O2) and hydroxyl radical (·OH) from cells (German, 1999). Cells produce and H2O2, resulting in unsaturated fatty acid peroxidation in animal tissues after slaughter (Kanner, 1994). Lipid oxidation is propagated under the catalytic actions of iron and other redox metal ions (Morrissey et al., 1998). However, the cytosol contains antioxidants that can suppress lipid oxidation in food and feed ingredients (Girotti, 1985). This process is particularly similar to the metabolism of ROS in human erythrocytes. Erythrocytes continuously produce by the autoxidation of haemoglobin (Cimen, 2008). The dismutation of generates H2O2 that can initiate lipid peroxidation in erythrocytes. Hydrogen peroxide reacts with heme Fe2+ to produce ·OH that can strengthen the process (Puppo and Halliwell, 1988). Our previous study demonstrated that exposure to FeSO4 and H2O2 triggers lipid oxidation in fish erythrocytes (Li et al., 2013). Similar to human erythrocytes, fish erythrocytes contain high concentrations of haemoglobin that can continuously produce ROS and a high content of unsaturated fatty acids that be easily oxidized by ROS in membranes (Li et al., 2016). The mechanisms of antioxidant defences in fish cells are similar to those in mammal cells (Winston and Giulioz, 1991). Moreover, fish erythrocytes retain the nucleus, mitochondria and other organelles, which are mostly similar to those of animal tissue cells in structure (Rothmann et al., 2000). Thus, it is possible that the fish erythrocyte system could be used as a model of lipid oxidation in food and feed ingredients.
Lipid oxidation leads to the breakdown of nutritional ingredients, change in taste, scent and colour, the development of toxic metabolites and a decrease in the shelf life of foods and feeds (Błaszczyk et al., 2013, Smet et al., 2008). Diets with oxidized lipid can result in decreased animal health, performance and quality (Chen et al., 2013, Han et al., 2012, Zhang et al., 2011). Thus, it is essential to expand our knowledge of how to protect foods and feeds against lipid oxidation. Synthetic antioxidants, such as butylated hydroxytoluene (BHT) and ethoxyquin (EQ), have been used for many years to stop lipid oxidation in foods and feeds (Błaszczyk et al., 2013). However, studies indicated that BHT and EQ are carcinogenic and toxic to animals (Ito et al., 1985, Nakagawa et al., 1994). Therefore, there is growing interest in replacing synthetic antioxidants with natural ingredients (Aksoy et al., 2013). Ginkgo biloba L. (Gb), a native plant of China, is now cultivated as an ornamental plant throughout the world. The standard extract of Gb leaves is well known as a natural antioxidative drug (Mahadevan and Park, 2008). However, a large amount of Gb leaves are treated as rubbish in many cities of China in the autumn and winter (Briancon-Scheid et al., 1983). Hence, it is possible to develop the extract of Gb leaves (EGbs) as an inhibitor of lipid oxidation in foods and feeds. However, information regarding the protective effects of the EGbs on food and feed materials is scarce.
In our previous study, ·OH induced apoptosis in fish erythrocytes, which provided a good model of oxidative damage in fish cells (Li et al., 2013). In this study, the apoptosis was induced in the same manner. We explored the effects of BHT and EQ and of ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) and aqueous extracts (AQE) of EGbs on lipid oxidation in a linoleic acid emulsion, fish flesh and fish feed and ·OH-treated carp erythrocytes. The purpose was to evaluate the protective effects of EGbs against lipid oxidation in food and feed ingredients for comparison with the effects of BHT and EQ. The results may provide a basis for applications of fish erythrocytes as a model of lipid oxidation and the use of EGbs as a natural antioxidant in foods and feeds.
2. Materials and methods
2.1. Chemicals
The BHT (analytical standard), heparin sodium (≥99%) and dimethyl sulfoxide (DMSO, ≥99.7%) were purchased from Sigma–Aldrich Co., LLC (St. Louis, MO, USA). The EQ (≥90%) was obtained from Shanghai PuZhen Biotech. Co., LTD (Shanghai, China). Ethyl ether, ethyl acetate, acetone and ethanol were of analytical grade and purchased from the Chengdu Kelong Chemical Reagent Factory (Chengdu, China). Aqueous solutions of H2O2 (30%) and FeSO4 (analytical grade) were obtained from the Shanghai Chemical Reagent Factory (Shanghai, China). Physiological carp saline (PCS) which contained (in mmol/L) 141.10 NaCl, 1.43 KCl, 0.99 CaCl2, 2.64 NaHCO3, and 6.16 glucose, was modified to obtain a total osmolarity of 280 mOsm/L and pH of 7.9 and was prepared in our laboratory. All other chemicals were analytical grade.
2.2. Preparation of EGbs
Leaves of Gb were collected in November from the trees growing near Neijiang Normal University (Neijiang, Sichuan, China). Botanical identification was performed in the Herbarium of the College of Life Sciences, where voucher samples were assigned a reference number and deposited. Prior to extraction following the methods of Wojcikowski et al. (2007), the dried leaves were ground to a powder (max particle size of 0.32 mm) using a Chinese medicine mill (Ronghao RHP-2000A, Zhejiang, China). Next, 50 g of the powder was extracted with 500 mL of ethyl ether, ethyl acetate, acetone, or ethanol or water at 20°C for 8 h using an Agitator (Dalong OS40-S, Beijing, China). The extraction using each solvent was repeated 3 times under the same conditions. After filtration, the solutions were removed and dried in vacuo by a rotary evaporator (Jinye RE-52CS, Shanghai, China) until a constant mass was achieved. The extraction process was repeated 4 times under the same conditions. The EEE, EAE, AE, EE and AQE were kept in sealed bottles in the dark and stored at −80°C until use.
2.3. Determination of total flavonoid content (TFC)
The TFC of EGbs was estimated using an aluminium chloride colorimetric assay (Zou et al., 2004).
2.4. Measurement of lipid oxidation in a linoleic acid emulsion
A mixture of 4 mL of absolute ethanol (control), EGbs, BHT or EQ (2 mg/mL), 4.1 mL of 2.51% linoleic acid in absolute ethanol, 8.0 mL of 0.02 mol/L phosphate buffer (pH 7.0), and 3.9 mL of distilled water in covered test tubes was placed in an oven at 45°C in the dark. After incubation for 24 h, the lipid oxidation in the emulsion was determined by measuring the absorbance of the resulting mixture at 500 and 532 nm based on the ferric thiocyanate (FTC) and thiobarbituric acid (TBA) methods, respectively (Sharma and Vig, 2014). The manoeuvre described above was repeated every 24 h until the absorbance of the control reached the maximum level. Four replicates were prepared for each treatment.
2.5. Measurement of lipid oxidation in fish flesh
Lipid oxidation in fish flesh was measured using the method described by Movileanu et al. (2013) with slight modifications. Healthy carp (100 to 110 g) obtained from local fisheries was anaesthetized and killed. Next, the head, fins and visceral organs were removed from the fish. The residual carcasses were homogenized in a meat grinder (Deming DM-JRJ10, Hangzhou, China) and extruded through a 3 mm die. The samples of fish flesh were individually blended and reground in a grinder (Ronghao RHP-2000A, Zhejiang, China) for 3 min and then extruded through a 1 mm die after 1.00 g/kg of dried BHT, EQ or EGbs in their original form was incorporated directly into the grinder, except for the control. Each sample (20 g) of fish flesh was placed in a separate 100 mL open beaker; the beakers were then transferred to an oven at 45°C for 8 d without stirring. Immediately after the storage period, lipid oxidation was measured by the TBA method. Four replicates were prepared for each treatment.
2.6. Measurement of lipid oxidation in fish feed
Eight types of feed were formulated for this experiment by the addition of 1.00 g/kg of dried BHT, EQ or EGbs based on the method of Lin and Zhou (2006) (Table 2). The feeds with particle-like properties were treated in the same manner as the fish flesh above. Lipid oxidation was measured by the TBA method. Four replicates were prepared for each treatment.
Table 2.
Composition of the experimental feeds containing butylated hydroxytoluene (BHT), ethoxyquin (EQ) or ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves.
| Item | Control | BHT | EQ | EEE | EAE | AE | EE | AQE |
|---|---|---|---|---|---|---|---|---|
| Ingredients, % | ||||||||
| Fish meal | 24.99 | 24.99 | 24.99 | 24.99 | 24.99 | 24.99 | 24.99 | 24.99 |
| Soybean meal | 30.21 | 30.21 | 30.21 | 30.21 | 30.21 | 30.21 | 30.21 | 30.21 |
| Wheat flour | 37.17 | 37.17 | 37.17 | 37.17 | 37.17 | 37.17 | 37.17 | 37.17 |
| Fish oil | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| Soybean oil | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 | 2.40 |
| Ca (H2PO4)2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Vitamin mixture1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Mineral mixture2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| BHT, EQ or extracts | 0.00 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| Nutrient content, % | ||||||||
| Crude protein | 34.10 | 34.10 | 34.10 | 34.10 | 34.10 | 34.10 | 34.10 | 34.10 |
| Crude lipid | 5.80 | 5.80 | 5.80 | 5.80 | 5.80 | 5.80 | 5.80 | 5.80 |
| Unsaturated fatty acids (n-3 + n-6) | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 | 2.42 |
Per kg of mineral mix: FeSO4·7H2O (20% Fe), 69.70 g; CuSO4·5H2O (25% Cu), 1.20 g; ZnSO4·7H2O (23% Zn), 21.64 g; MnSO4·H2O (32% Mn), 4.09 g; Na2SeO3·5H2O (1% Se), 2.50 g; KI (4% I), 2.90 g; CaCO3, 897.98 g.
Per kg of vitamin mix: retinyl acetate (500,000 IU/g), 0.80 g; cholecalciferol (500,000 IU/g), 0.48 g; DL-α-tocopherol acetate (50%), 20.00 g; menadione (23%), 0.43 g; thiamin nitrate (90%), 0.11 g; riboflavine (80%), 0.63 g; pyridoxine HCl (81%), 0.92 g; cyanocobalamin (1%), 0.10 g; ascorhyl acetate (93%), 7.16 g; D-calcium pantothenate (90%), 2.73 g; niacin (99%), 2.82 g; D-biotin (2%), 5.00 g; meso-inositol (99%), 52.33 g; folic acid (96%), 0.52 g.
Inhibition (I) of lipid oxidation in the linoleic acid emulsion, fish flesh and feeds was calculated using the following equation: I (%) = 100 × (1−A1/A0). Here, A0 was the absorbance of the control, and A1 was the absorbance of the sample solution.
2.7. Cytoprotection assays
2.7.1. Experimental procedure
The experimental procedures were based on those described by Li et al. (2016), with slight modifications. The BHT, EQ, EEE, EAE, AE, EE or AQE was dissolved in PCS containing 1% erythrocytes (vol/vol) and 0.1% DMSO (vol/vol) to obtain a final concentration of 0.25 mg/mL. For the positive and control groups, BHT, EQ and EGbs was not added to the PCS, but the PCS did contain 1% erythrocytes (vol/vol) and 0.1% DMSO (vol/vol). After all the above treatments were pre-incubated at 37°C for 3 h, FeSO4 and H2O2 was respectively added at a final concentration of 40 and 20 μmol/L for the induction of apoptosis, except for the control group. After incubation at 37°C for 6 h, the samples were centrifuged for 3 min (1,000 × g, 4°C). The erythrocytes were collected to measure the levels of , H2O2, malonaldehyde (MDA) and protein carbonyl (PC), the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx), and the phosphatidylserine (PS) exposure and DNA fragmentation. The experiment was performed with 4 replicates per treatment and the control.
All procedures above were approved by the Institutional Animal Care and Use Committee of Neijiang Normal University in accordance with the Institutional Ethics Committee of the Chinese Institute of Chemical Biology guidelines.
2.7.2. Biochemical analysis
The contents of , H2O2, MDA and PC in the erythrocytes were measured as described by Li et al. (2013). The activities of SOD, CAT and GPx were determined by the method of Fan et al. (2015). The protein concentration was determined by the method of Darbkin (1946).
2.7.3. Measurement of apoptosis
The PS exposure and DNA fragmentation in fish erythrocytes were respectively assessed by the Annexin V-FITC Apoptosis Detection Kit and TdT-mediated dUTP nick end labeling (TUNEL) Apoptosis Assay Kit (Beyotime, Nantong, China) as described previously (Li et al., 2016).
2.8. Statistical analysis
The data are expressed as the means ± standard deviation (SD). The data were subjected to one-way analysis of variance (ANOVA). Duncan's multiple range test was used to determine significant differences. The significance level was 95% (α = 0.05). The statistical analysis was performed using SPSS 13.0 for Windows (Chicago, IL, USA).
3. Results
3.1. Effects of BHT, EQ and EGbs on the oxidation of linoleic acid
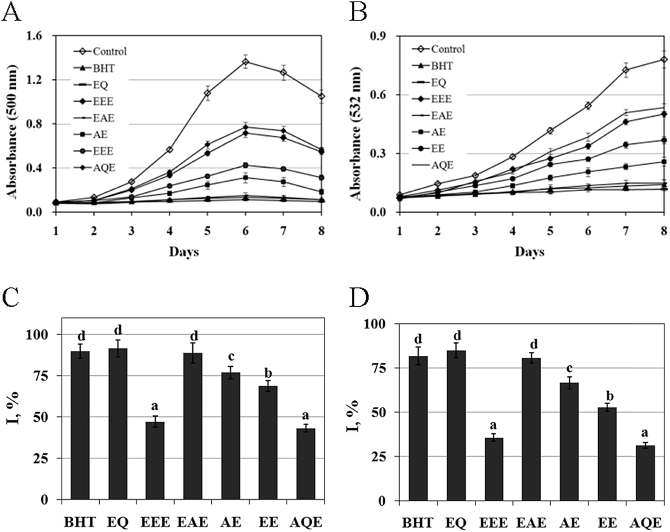
As shown in Fig. 1A, the oxidation of linoleic acid was manifested in the increase of the absorption at 500 nm measured for the FTC method on d 6 in the control group (P < 0.05). However, BHT, EQ and EGbs significantly blunted the increase in the absorbance at 500 nm (P < 0.05). Of the examined compounds, BHT, EQ and EAE showed the strongest inhibitory effects on the oxidation of linoleic acid (Fig. 1C). The rank order of the inhibition efficacy was EQ = BHT = EAE > AE > EE > EEE = AQE (P < 0.05).
Fig. 1.
Influence of butylated hydroxytoluene (BHT) or ethoxyquin (EQ) and of ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves on absorbance value at 500 nm for the FTC method (A) and at 532 nm for the TBA method (B). The inhibition (I) induced by these compounds on the lipid oxidation measured using the FTC method (C) and the TBA method (D) in a linoleic acid emulsion. The data represent the means ± SD of 4 replicates. a–d Bars with different superscripts are significantly different (P < 0.05).
The oxidation of linoleic acid was demonstrated by the increase in the absorption at 532 nm measured for the TBA method on d 8 in the control group (Fig. 1B) (P < 0.05). However, BHT, EQ and EGbs significantly blunted the increase in the absorbance at 532 nm (P < 0.05), suggesting a decrease in the oxidation of linoleic acid. Among the examined compounds, BHT, EQ and EAE showed the strongest inhibitory effects (Fig. 1D). The rank order of the inhibition efficacy agreed approximately with that found using the FTC method above (P < 0.05).
3.2. Effects of BHT, EQ and EGbs on lipid oxidation in fish flesh and feed
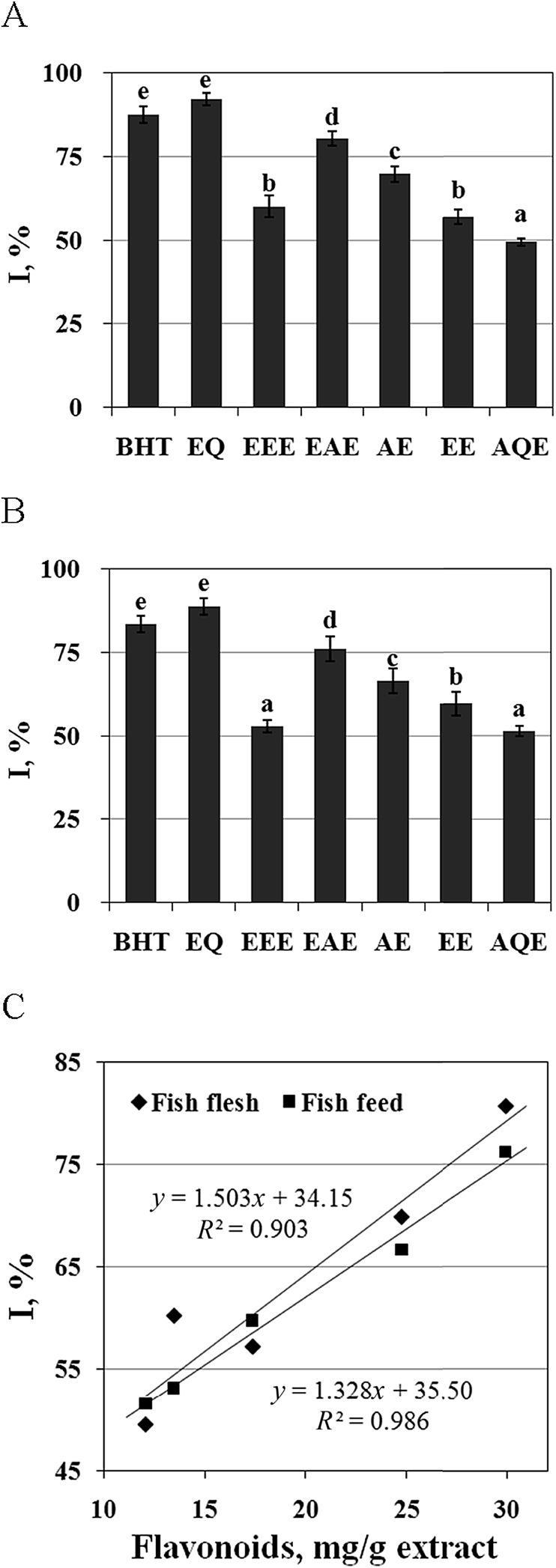
As shown in Fig. 2, BHT, EQ and EGbs significantly inhibited lipid oxidation in fish flesh and feed (P < 0.05). In particular, when the fish flesh and feed were treated with BHT or EQ, the inhibition reached the highest level for the examined compounds. The inhibitory effects of EAE in fish flesh and feed on the lipid oxidation were respectively estimated to be 80.62% and 76.14%, which are the maximum values for the examined extracts. The inhibition efficacy in fish flesh decreased in the order EQ = BHT > EAE > AE > EEE = EE > AQE (P < 0.05) (Fig. 2A). The rank order of the inhibition efficacy in fish feed was EQ = BHT > EAE > AE > EE > EEE = AQE (P < 0.05) (Fig. 2B). The inhibitory effects of EEE, EAE, AE, EE and AQE on the lipid oxidation in fish feed and fish flesh were positively correlated with their flavonoid content (P < 0.05) (Fig. 2C) (Table 1).
Fig. 2.
Inhibition (I) caused by butylated hydroxytoluene (BHT) or ethoxyquin (EQ) and by ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves on the lipid oxidation in fish flesh (A) and fish feed (B). Their correlation (C) with the flavonoid content in the extracts. The data represent the means ± SD of 4 replicates. a–e Bars with different superscripts are significantly different (P < 0.05).
Table 1.
Yield rate and flavonoid content in the ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) and aqueous extracts (AQE) of Ginkgo biloba leaves.
| Extracts | Yield, g/kg dry herb | Flavonoids, mg/g dry extracts |
|---|---|---|
| EEE | 203.91 ± 9.56e | 13.44 ± 0.99a |
| EAE | 157.19 ± 10.01d | 29.90 ± 2.13d |
| AE | 107.94 ± 7.28c | 24.75 ± 1.87c |
| EE | 56.12 ± 4.04a | 17.34 ± 1.13b |
| AQE | 85.53 ± 6.16b | 12.08 ± 0.86a |
a–e Within a same column, values with different superscripts are significantly different (P < 0.05), and the data represent the means ± SD of 4 replicates.
3.3. Effects of BHT, EQ and EGbs on the ·OH-treated carp erythrocytes
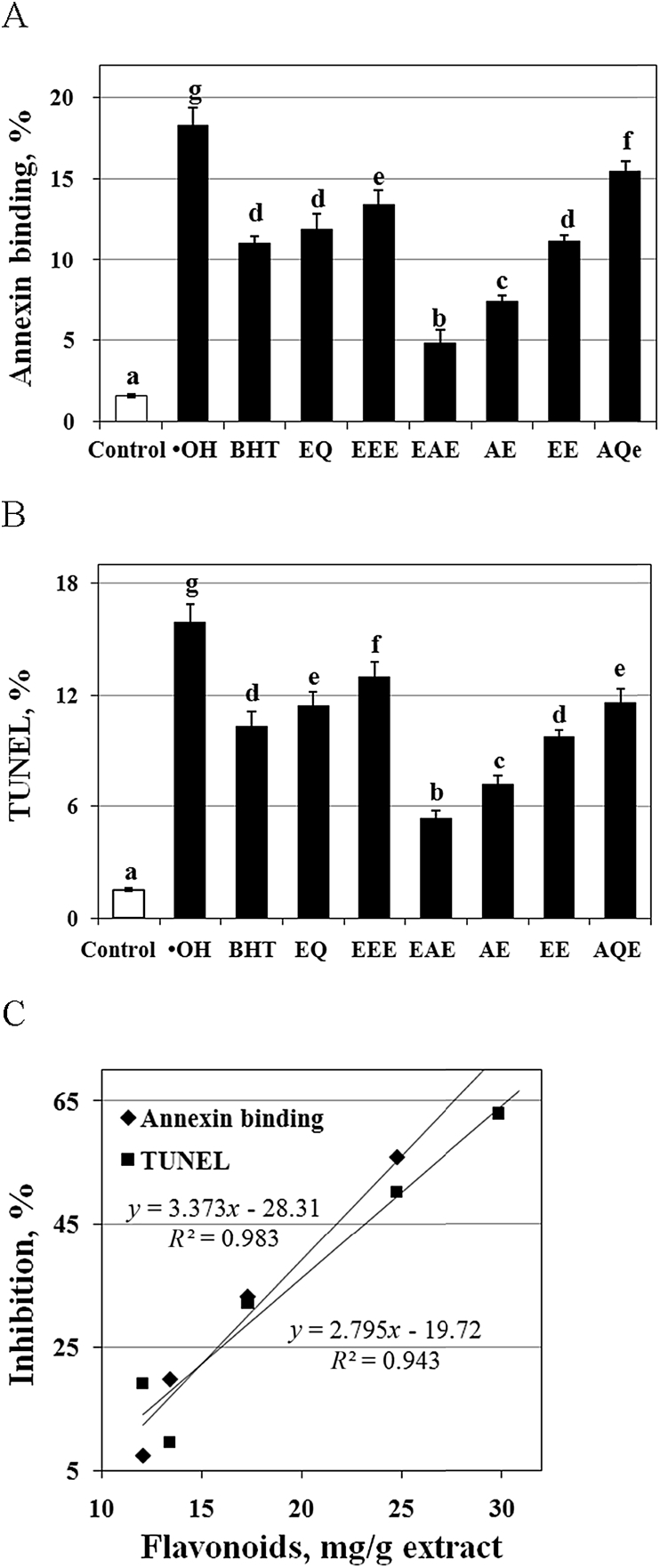
As shown in Fig. 3, the levels of annexin binding and TUNEL-positive cells were significantly increased in the carp erythrocytes exposed to ·OH alone (P < 0.05), suggesting an increase in PS exposure and DNA fragmentation in the control group. However, treatment with BHT, EQ and EGbs significantly decreased the levels of annexin binding (Fig. 3A) and TUNEL-positive (Fig. 3B) cells (P < 0.05), suggesting a decrease in the PS exposure and DNA fragmentation in ·OH-treated carp erythrocytes. In particular, when the erythrocytes were treated with EAE in the presence of ·OH, the level of annexin binding and TUNEL-positive cells was estimated to be the minimum value for all examined compounds. The levels of PS exposure and DNA fragmentation in the ·OH-treated erythrocytes treated with AE were lower than those for the BHT and EQ treatments. The inhibitory effects of EEE, EAE, AE, EE and AQE on the ·OH-induced apoptosis were positively correlated with their flavonoid content (P < 0.05) (Fig. 3C) (Table 1).
Fig. 3.
Effects of butylated hydroxytoluene (BHT) or ethoxyquin (EQ) and of ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves on annexin binding (A) and TUNEL (B) in ·OH-treated carp erythrocytes. Correlation (C) between anti-apoptotic function and flavonoid content in the extracts of Ginkgo biloba leaves. The data represent the means ± SD of 4 replicates. a–g Bars with different superscripts are significantly different (P < 0.05).
The effects of BHT, EQ and EGbs on , H2O2, MDA and PC in ·OH-treated carp erythrocytes are presented in Table 3. Exposure to ·OH significantly increased the levels of , H2O2, MDA and PC in the erythrocytes relative to those in the untreated control (P < 0.05). However, treatment with BHT, EQ and EGbs effectively prevented the increase in , H2O2, MDA and PC levels in the erythrocytes that had been exposed to ·OH (P < 0.05). In particular, the ·OH-treated erythrocytes that were also treated with BHT and EQ showed the minimum values of , H2O2, MDA and PC for all examined compounds. The levels of and MDA for the EAE treatment were equivalent to those for BHT. Additionally, the levels of H2O2 and PC for the EAE treatment were estimated to be the minimum value for all examined extracts. The inhibition order of BHT, EQ and EGbs on and H2O2 were EQ = BHT = EAE > AE > EE = AQE > EEE and EQ = BHT > EAE > AE > EE > AQE = EEE, respectively (P < 0.05). The inhibition order of BHT, EQ and EGbs on MDA and PC agreed approximately with those for and H2O2 above (P < 0.05).
Table 3.
Effects of butylated hydroxytoluene (BHT) or ethoxyquin (EQ) and of ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves on the levels of superoxide anion (), hydrogen peroxide (H2O2), malonaldehyde (MDA) and protein carbonyl (PC) in ·OH-treated carp erythrocytes.
| Treatment | , U/g protein | H2O2, μmol/g protein | MDA, nmol/mg protein | PC, nmol/mg protein |
|---|---|---|---|---|
| Control | 23.32 ± 1.60a | 34.87 ± 1.65a | 1.52 ± 0.10a | 0.87 ± 0.06a |
| ·OH | 59.01 ± 4.34g | 96.88 ± 7.16g | 3.29 ± 0.15g | 2.17 ± 0.13g |
| ·OH + BHT | 29.87 ± 1.87bc | 40.96 ± 1.77b | 1.81 ± 0.10bc | 1.09 ± 0.06b |
| ·OH + EQ | 27.60 ± 1.78b | 39.25 ± 2.96b | 1.74 ± 0.10b | 0.98 ± 0.05ab |
| ·OH + EEE | 53.26 ± 2.55f | 76.76 ± 5.34f | 2.92 ± 0.17f | 2.04 ± 0.07f |
| ·OH + EAE | 32.63 ± 1.52c | 47.72 ± 3.14c | 1.95 ± 0.07c | 1.32 ± 0.08c |
| ·OH + AE | 38.48 ± 1.87d | 61.95 ± 3.80d | 2.28 ± 0.18d | 1.58 ± 0.06d |
| ·OH + EE | 46.16 ± 2.62e | 69.63 ± 4.58e | 2.66 ± 0.12e | 1.80 ± 0.14e |
| ·OH + AQE | 48.12 ± 2.89e | 76.87 ± 4.98f | 3.03 ± 0.09f | 1.99 ± 0.07f |
a–g Within a same column, values with different superscripts are significantly different (P < 0.05), and the data represent the means ± SD of 4 replicates.
As presented in Table 4, the activities of SOD, CAT and GPx were markedly decreased in carp erythrocytes exposed to ·OH alone (P < 0.05). However, for the BHT, EQ and EGbs treatments, the decreases were effectively inhibited in the erythrocytes that had been exposed to ·OH (P < 0.05). The activities of SOD, CAT and GPx in the ·OH-treated carp erythrocytes that were also treated with EQ were the highest values compared with those of the examined compounds. The activities of CAT and GPx for the BHT treatment were estimated to be equivalent to those of EQ. The CAT activity for the EAE treatment was estimated to be 5.11 U/mg proteins, which is equivalent to that of BHT. Additionally, the activities of SOD and GPx for the EAE treatment were estimated to be the maximum values for the examined extracts.
Table 4.
Effects of butylated hydroxytoluene (BHT) or ethoxyquin (EQ) and of ethyl ether extracts (EEE), ethyl acetate extracts (EAE), acetone extracts (AE), ethanol extracts (EE) or aqueous extracts (AQE) of Ginkgo biloba leaves on the activities of superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) in ·OH-treated carp erythrocytes.
| Treatment | SOD, U/mg protein | CAT, U/mg protein | GPx, U/mg protein |
|---|---|---|---|
| Control | 44.50 ± 1.63g | 6.05 ± 0.33g | 65.07 ± 4.60g |
| ·OH | 9.37 ± 0.28a | 3.17 ± 0.23a | 26.68 ± 1.69a |
| ·OH + BHT | 39.58 ± 2.24f | 5.40 ± 0.27ef | 57.71 ± 2.20f |
| ·OH + EQ | 42.31 ± 2.23g | 5.82 ± 0.42fg | 60.72 ± 3.31f |
| ·OH + EEE | 18.86 ± 1.48b | 3.63 ± 0.18b | 38.80 ± 1.71c |
| ·OH + EAE | 35.85 ± 2.06e | 5.11 ± 0.29e | 52.50 ± 2.51e |
| ·OH + AE | 29.70 ± 1.99d | 4.52 ± 0.28d | 47.35 ± 2.79d |
| ·OH + EE | 24.19 ± 1.31c | 4.03 ± 0.29bc | 41.89 ± 2.13c |
| ·OH + AQE | 17.68 ± 1.03b | 4.15 ± 0.26cd | 33.43 ± 1.85b |
a–g Within a same column, values with different superscripts are significantly different (P < 0.05), and the data represent the means ± SD of 4 replicates.
4. Discussion
4.1. BHT, EQ and EGbs inhibited lipid oxidation in the linoleic acid emulsion, fish flesh and feed
The autoxidation of unsaturated fatty acids is an important factor leading to the lipid oxidation in food and feed materials (Girotti, 1985). Thus, a linoleic acid emulsion has been used as a food system model to investigate lipid oxidation (Yuan et al., 2005). The FTC method is used to evaluate the amount of peroxide at the initial stage of lipid oxidation when the peroxide reacts with FeCl2 to form a reddish ferric chloride pigment that can be measured at 500 nm. In contrast, the TBA method is used to measure the levels of MDA, which is a degradation product of peroxide at the later stage of lipid oxidation when MDA binds TBA to form a red complex that can be measured at 532 nm (Sharma and Vig, 2014). In this study, BHT, EQ and EGbs significantly blunted the increase in the absorbance at 500 nm, suggesting a decrease in the amount of peroxide, and they reduced the absorbance at 532 nm, suggesting a decrease in the MDA levels in the linoleic acid emulsion. This finding is consistent with the report that BHT, EQ and the AQE and EE of Gb leaves suppress the oxidation of linoleic acid (Gulcin, 2006, Kobus et al., 2009, Marco, 1968). In particular, of all the examined EGbs, EAE showed the strongest inhibitory effect, which was equivalent to the effect of BHT and EQ. This result suggested that BHT, EQ and EGbs can inhibit the oxidation of unsaturated fatty acids during the entire process of lipid oxidation.
The reaction of MDA with TBA in animal tissues is same as in the case of the linoleic acid emulsion (Ohkawa et al., 1979). In the present study, BHT, EQ and EGbs significantly protected against the formation of MDA in fish flesh and feed, suggesting inhibition of lipid oxidation. Among all examined EGbs, EAE showed the strongest effects. This finding is in agreement with the report that BHT and EQ suppress lipid oxidation in foods and feeds (Błaszczyk et al., 2013, Sanhueza et al., 2000). No reports have been published indicating that EGbs prevents lipid oxidation in food and feed materials. The results of this study suggested that BHT, EQ and EGbs can inhibit lipid oxidation in food and feed materials. The antioxidant effects of EGbs may be closely associated with their flavonoid content. It has been reported that the plant-derived flavonoids in Gb possess potent antioxidant activity (Mahadevan and Park, 2008). In this study, a positive correlation was found between the antioxidant effects and the flavonoid content in EGbs. This result suggested that the antioxidant activity of EGbs may be caused by the presence of flavonoids in foods and feeds.
4.2. BHT, EQ and EGbs inhibited ·OH-induced apoptosis in carp erythrocytes
The PS exposure and DNA fragmentation are biomarkers of apoptosis (Li et al., 2015, Tan et al., 2010). In this study, BHT, EQ and EGbs effectively inhibited the PS exposure and DNA fragmentation induced by ·OH in carp erythrocytes. These results confirmed that BHT, EQ and EGbs could protect against ·OH-induced apoptosis in fish erythrocytes. In particular, the inhibitory effects of EAE and AE on the apoptosis were stronger than those of BHT and EQ. This finding is consistent with the reports that EGb761, a standard extract from the leaves of Gb, has a protective effect against apoptosis in human keratinocytes (Zhu et al., 2005). However, data on the anti-apoptosis properties of BHT and EQ in animal cells are scarce.
There may be a positive linking the anti-apoptosis effects of BHT, EQ and EGbs to ROS in fish erythrocytes. It is has been reported that ROS can trigger apoptosis in animal cells (Shen and Liu, 2006, Yin et al., 2015). The autoxidation of haemoglobin can continuously produce , and the dismutation of can generate H2O2 in human erythrocytes (Cimen, 2008). In the present study, the levels of and H2O2 increased in the carp erythrocytes exposed to ·OH. This finding is in line with the cases in which cells in animal tissues produce and H2O2 in the immediate post–slaughter period (Kanner, 1994). However, treatment with BHT, EQ and EGbs effectively decreased the levels of and H2O2 in carp erythrocytes exposed to ·OH. Among the examined EGbs, EAE showed the strongest effects. Moreover, the inhibitory effects of EAE on were almost equivalent to those of BHT. This finding suggested that BHT, EQ and EGbs can decrease the generation of ROS in fish erythrocytes, and it was consistent with the reports showing that EGb761 can scavenge ROS in rat lymphocytes (Eckert et al., 2003). These results demonstrated that BHT, EQ and EGbs could protect fish erythrocytes against apoptosis by inhibiting the generation of ROS.
The anti-apoptosis effects of BHT, EQ and EGbs may be closely associated with the oxidation of cellular components in fish erythrocytes. The oxidative products of lipids and proteins play an important role in the induction of apoptosis in mammalian cells (Li et al., 2016). Hydrogen peroxide can react with heme Fe2+ to produce ·OH in human erythrocytes (Cimen, 2008). Hydroxyl radical can oxidize cellular components, such as lipids and proteins, leading to the formation of MDA and PC (Li et al., 2013, Yin et al., 2016). In this study, ·OH induced an increase in the MDA and PC levels in carp erythrocytes. This finding suggested that ·OH induces the oxidation of lipids and proteins in carp erythrocytes, and it is in good agreement with the reports showing that ROS results in lipid oxidation with a subsequent increase in MDA levels in animal tissues during handling, processing, storage and cooking (Morrissey et al., 1998). However, BHT, EQ and EGbs markedly decreased the MDA and PC levels in ·OH-treated carp erythrocytes. Among all examined extracts, EAE showed the strongest effects on MDA and PC. Moreover, the inhibitory effects of EAE on MDA were almost equivalent to those of BHT. This finding suggested that BHT, EQ and EGbs can quench the oxidation of lipids and proteins in ·OH-treated carp erythrocytes. This result is consistent with the reports showing that BHT and EQ respectively decrease lipid peroxidation in cerebellar granule cells (Puttfarcken et al., 1993) and rat hepatic microsomes (Nilsson et al., 1989), and EGb761 reduces the lipid oxidation in bovine endothelial cells (Rong et al., 1996). These studies demonstrated that BHT, EQ and EGbs could protect fish erythrocytes from apoptosis by preventing the oxidation of cellular components.
The cytosol contains antioxidants that can scavenge intracellular ROS and suppress lipid oxidation in cells (Wu et al., 2011). The key enzymatic antioxidants in aquatic organisms are SOD, CAT and GPx (Li et al., 2013). The SOD and CAT can inhibit apoptosis in human neutrophils (Sulowska et al., 2005). Thus, the anti-apoptosis activity of BHT, EQ and EGbs may be related to the antioxidants in fish erythrocytes. In the present study, BHT, EQ and EGbs restored the activities of SOD, CAT and GPx in carp erythrocytes, which were depressed following ·OH exposure. Among all examined extracts, EAE showed the strongest effects. Moreover, the protective effects of EAE on CAT were almost equivalent to those of BHT. This finding is in good harmony with the report showing that EGb761 prevents the decline of SOD activities in human keratinocytes (Zhu et al., 2005). No reports have been published on the effects of BHT and EQ on enzymatic antioxidants in animal cells. These results revealed that in addition to quenching ROS and preventing the oxidation of cellular components, BHT, and EQ and EGbs may affect apoptosis through their elevation of enzymatic antioxidant activity. Moreover, the present study showed that the inhibition order of EEE, EAE, AE, EE and AQE on the generation of ROS and oxidation of cellular components agreed approximately with that for the lipid oxidation in a linoleic acid emulsion, fish flesh and fish feed. In our experience, fish erythrocytes are easy to harvest and culture in vitro (Li et al., 2016). These results revealed that the fish erythrocyte system could be used as a model of the lipid oxidation in food and feed ingredients or materials. Among the examined extracts, the EAE showed the strongest effects on decreasing the generation of ROS, inhibiting the oxidation of cellular components and restoring the activities of antioxidants. Thus, the EAE may be used as a natural antioxidant in food and feed materials.
The anti-apoptosis effect of EGbs may be closely associated with the flavonoid content. One of the most active ingredients is flavonoids in Gb leaves, which can scavenge apoptosis-inducing factors, such as metal ions, ROS and oxidized lipid (Mahadevan and Park, 2008). In the present study, the inhibitory effects of EGbs on the ·OH-induced apoptosis were positively correlated with their flavonoid content. This result demonstrated that the anti-apoptotic effects in EGbs may be due to the presence of flavonoids. This idea is in agreement with the reports showing that flavonoids prevent toxin-induced apoptosis in rat hepatocytes (Blankson et al., 2000). Flavonoids in the aglycone form can be absorbed directly in the intestine in humans (Goh and Barlow, 2004). Unabsorbed flavonoids in the glycosidic form may be metabolized by bacterial enzymes in the colon, and then absorbed and transported to the liver (Mahadevan and Park, 2008). In this study, the EAE possessed the highest flavonoid content and showed the most anti-oxidative and anti-apoptotic effects. Thus, it is possible that the EAE could be used as a natural antioxidant and apoptosis inhibitor in fish.
5. Conclusion
In summary, our study first showed that the EEE, EAE, AE, EE and AQE of Gb leaves inhibit both lipid oxidation in food and feed materials and ·OH-induced apoptosis in fish erythrocytes. Of all the examined EGbs, the EAE showed the strongest effects. The effects of EAE on the oxidation of linoleic acid were equivalent to those of BHT and EQ, and the effects on , MDA and CAT were equivalent to those of BHT, and the effects on apoptosis were stronger than those of BHT and EQ. Therefore, the EAE could be used as a potential natural antioxidant and apoptosis inhibitor. Furthermore, we found that the inhibition order of EGbs on the generation of ROS and oxidation of cellular components in fish erythrocytes agreed approximately with that for the lipid oxidation in food and feed materials. Thus, the fish erythrocyte system can be used as an experimental model of lipid oxidation in food and feed ingredients. This study is the first to reveal that the anti-oxidative activities and anti-apoptotic effects of EGbs may be due to the presence of flavonoids.
Acknowledgements
This research was financially supported by the Doctoral Research Fund of Neijiang Normal University (14B07). The authors wish to thank the personnel of these teams for their kind assistance.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aksoy L., Kolay E., Agilonu Y., Aslan Z., Kargioglu M. Free radical scavenging activity, total phenolic content, total antioxidant status, and total oxidant status of endemic Thermopsis turcica Saudi. J Biol Sci. 2013;20:235–239. doi: 10.1016/j.sjbs.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankson H., Grotterùd E., Seglen P. Prevention of toxin-induced cytoskeletal disruption and apoptotic liver cell death by the grapefruit flavonoid, naringin. Cell Death Differ. 2000;7:739–746. doi: 10.1038/sj.cdd.4400705. [DOI] [PubMed] [Google Scholar]
- Błaszczyk A., Augustyniak A., Skolimowski J. Ethoxyquin: an antioxidant used in animal feed. Int J Food Sci. 2013;2013:1–12. doi: 10.1155/2013/585931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briancon-Scheid F., Lobstein-Guth A., Anton R. HPLC separation and quantitative determination of biflavones in leaves from Ginkgo biloba. Planta Med. 1983;49:204–207. doi: 10.1055/s-2007-969851. [DOI] [PubMed] [Google Scholar]
- Chen Y.J., Liu L.J., Tian L.X., Niu J., Liang G.Y., Yang H.J. Effect of dietary vitamin E and selenium supplementation on growth, body composition, and antioxidant defense mechanism in juvenile largemouth bass (Micropterus salmoide) fed oxidized fish oil. Fish Physiol Biochem. 2013;39:593–604. doi: 10.1007/s10695-012-9722-1. [DOI] [PubMed] [Google Scholar]
- Cimen M.Y. Free radical metabolism in human erythrocytes. Clin Chim Acta. 2008;390:1–11. doi: 10.1016/j.cca.2007.12.025. [DOI] [PubMed] [Google Scholar]
- Darbkin D.L. Spectrophotometric studies. XIV the crystallographic and optical properties of the hemoglobin of man in comparison with these of other species. J Biol Chem. 1946;164:703–772. [PubMed] [Google Scholar]
- Eckert A., Keil U., Kressmann S., Schindowski K., Leutner S., Leutz S. Effects of EGb 761 Ginkgo biloba extract on mitochondrial function and oxidative stress. Pharmacopsychiatry. 2003;36(Suppl. 1):S15–S23. doi: 10.1055/s-2003-40449. [DOI] [PubMed] [Google Scholar]
- Fan Z., Xiao Y., Chen Y., Wu X., Zhang G., Wang Q. Effects of catechins on litter size, reproductive performance and antioxidative status in gestating sows. Animal Nutrition. 2015;1(4):271–275. doi: 10.1016/j.aninu.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsche K.L., Johnston P.V. Rapid autoxidation of fish oil in diets without added antioxidants. J Nutr. 1988;118:425–426. doi: 10.1093/jn/118.4.425. [DOI] [PubMed] [Google Scholar]
- German J.B. Food processing and lipid oxidation. Adv Exp Med Biol. 1999;459:23–50. doi: 10.1007/978-1-4615-4853-9_3. [DOI] [PubMed] [Google Scholar]
- Girotti A.W. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1:87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Goh L.M.L., Barlow P.J. Flavonoid recovery and stability from Ginkgo biloba subjected to a simulated digestion process. Food Chem. 2004;86:195–202. [Google Scholar]
- Gulcin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Han Y.Z., Ren T.J., Jiang Z.Q., Jiang B.Q., Gao J., Koshio S. Effects of palm oil blended with oxidized fish oil on growth performances, hematology, and several immune parameters in juvenile Japanese sea bass, Lateolabrax japonicas. Fish Physiol Biochem. 2012;38:1785–1794. doi: 10.1007/s10695-012-9675-4. [DOI] [PubMed] [Google Scholar]
- Ito N., Fukushima S., Tsuda H. Carcinogenicity and modification of the carcinogenic response BHA, BHT, and other antioxidants. Crit Rev Toxicol. 1985;15:109–150. doi: 10.3109/10408448509029322. [DOI] [PubMed] [Google Scholar]
- Kanner J. Oxidative processes in meat and meat products: quality implications. Meat Sci. 1994;36:169–189. doi: 10.1016/0309-1740(94)90040-X. [DOI] [PubMed] [Google Scholar]
- Kobus J., Flaczyk E., Siger A., Nogala-Kałucka M., Korczak J., Pegg R.B. Phenolic compounds and antioxidant activity of extracts of Ginkgo leaves. Eur J Lipid Sci Technol. 2009;111:1150–1160. [Google Scholar]
- Li H.T., Feng L., Jiang W.D., Liu Y., Jiang J., Li S.H. Oxidative stress parameters and anti-apoptotic response to hydroxyl radicals in fish erythrocytes: protective effects of glutamine, alanine, citrulline and proline. Aquat Toxicol. 2013;126:169–179. doi: 10.1016/j.aquatox.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Li H.T., Feng L., Jiang W.D., Liu Y., Jiang J., Zhang Y.A. Ca(2+) and caspases are involved in hydroxyl radical-induced apoptosis in erythrocytes of Jian carp (Cyprinus carpio var. Jian) Fish Physiol Biochem. 2015;41:1305–1319. doi: 10.1007/s10695-015-0087-0. [DOI] [PubMed] [Google Scholar]
- Li H.T., Jiang W.D., Liu Y., Jiang J., Zhang Y.A., Wu P. The metabolites of glutamine prevent hydroxyl radical-induced apoptosis through inhibiting mitochondria and calcium ion involved pathways in fish erythrocytes. Free Radic Biol Med. 2016;92:126–140. doi: 10.1016/j.freeradbiomed.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Lin Y., Zhou X.Q. Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian carp (Cyprinus carpio var. Jian) Aquaculture. 2006;256:389–394. [Google Scholar]
- Mahadevan S., Park Y. Multifaceted therapeutic benefits of Ginkgo biloba L.: chemistry, efficacy, safety, and uses. J Food Sci. 2008;73:R14–R19. doi: 10.1111/j.1750-3841.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- Marco G.J. A rapid method for evaluation of antioxidants. J Am Oil Chemists' Soc. 1968;45:594–598. [Google Scholar]
- Morrissey P.A., Sheehy P.J.A., Galvin K., Kerry J.P., Buckley D.J. Lipid stability in meat and meat products. Meat Sci. 1998;49:S73–S86. [PubMed] [Google Scholar]
- Movileanu I., Núñez de González M.T., Hafley B., Miller R.K., Keeton J.T. Comparison of dried Plum puree, Rosemary extract, and BHA/BHT as antioxidants in irradiated ground beef patties. Int J Food Sci. 2013;2013:1–7. doi: 10.1155/2013/360732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Yaguchi K., Suzuki T. Comparative cytotoxicity between butylated hydroxytoluene and its methylcarbamate derivative, terbucarb, on isolated rat hepatocytes. Bull Environ Contam Toxicol. 1994;52:511–515. doi: 10.1007/BF00194137. [DOI] [PubMed] [Google Scholar]
- Nilsson U.A., Olsson L.I., Carlin G., Bylund-Fellenius A.C. Inhibition of lipid peroxidation by spin labels relationships between structure and function. J Biol Chem. 1989;264:11131–11135. [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Biochem J. 1988;249:185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttfarcken P.S., Getz R.L., Coyle J.T. Kainic acid-induced lipid peroxidation: protection with butylated hydroxytoluene and U78517F in primary cultures of cerebellar granule cells. Brain Res. 1993;624:223–232. doi: 10.1016/0006-8993(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Rong Y.Q., Geng Z.H., Lau B.H.S. Ginkgo biloba attenuates oxidative stress in macrophages and endothelial cells. Free Radic Biol Med. 1996;20:121–127. doi: 10.1016/0891-5849(95)02016-0. [DOI] [PubMed] [Google Scholar]
- Rothmann C., Levinshal T., Timan B., Avtalion R.R., Malik Z. Spectral imaging of red blood cells in experimental anemia of Cyprinus carpio. Comp Biochem Physiol Part A. 2000;125:75–83. doi: 10.1016/s1095-6433(99)00157-9. [DOI] [PubMed] [Google Scholar]
- Sanhueza J., Nieto S., Valenzuela A. Thermal stability of some commercial synthetic antioxidants. J Am Oil Chemists' Soc. 2000;77:933–936. [Google Scholar]
- Sharma S., Vig A.P. vol. 2014. BioMed Research International; 2014. pp. 1–8. (Preliminary phytochemical screening and in vitro antioxidant activities of Parkinsonia aculeata Linn). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H.M., Liu Z.G. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- Smet K., Raes K., Huyghebaert G., Haak L., Arnouts S., De Smet S. Lipid and protein oxidation of broiler meat as influenced by dietary natural antioxidant supplementation. Poult Sci. 2008;87:1682–1688. doi: 10.3382/ps.2007-00384. [DOI] [PubMed] [Google Scholar]
- Sulowska Z., Majewska E., Klink M., Banasik M., Tchorzewski H. Flow cytometric evaluation of human neutrophil apoptosis during nitric oxide generation in vitro: the role of exogenous antioxidants. Mediat Inflamm. 2005;2005:81–87. doi: 10.1155/MI.2005.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Yin Y., Kong X., Li P., Li X., Gao H. L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–1235. doi: 10.1007/s00726-009-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston G.W., Giulioz R.T.D. Prooxidant and Antioxidant in aquatic organisms. Aquat Toxicol. 1991;19:137–161. [Google Scholar]
- Wojcikowski K., Stevenson L., Leach D., Wohlmuth H., Gobe G. Antioxidant capacity of 55 medicinal herbs traditionally used to treat the urinary system: a comparison using a sequential three-solvent extraction process. J Altern Complement Med. 2007;13:103–109. doi: 10.1089/acm.2006.6122. [DOI] [PubMed] [Google Scholar]
- Wqsowicz E., Gramza A., Hêoe M., Jeleñ H.H., Korczak J., Malecka M. Oxidation of lipids in food. Pol J Food Nutr Sci. 2004;13:87–100. [Google Scholar]
- Wu C.L., Zhang W.B., Mai K.S., Xu W., Zhong X.L. Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comp Biochem Physiol C. 2011;154:1–6. doi: 10.1016/j.cbpc.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Yin J., Duan J., Cui Z., Ren W., Li T., Yin Y. Hydrogen peroxide-induced oxidative stress activates NF-κB and Nrf2/Keap1 signals and triggers autophagy in piglets. RSC Adv. 2015;5:15479–15486. [Google Scholar]
- Yin J., Ren W., Yang G., Duan J., Huang X., Fang R. l-Cysteine metabolism and its nutritional implications. Mol Nutr Food Res. 2016;60:134–146. doi: 10.1002/mnfr.201500031. [DOI] [PubMed] [Google Scholar]
- Yuan Y.V., Bone D.E., Carrington M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005;91:485–494. doi: 10.1016/j.fct.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J Agric Food Chem. 2011;59:969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]
- Zhu Q.X., Shen T., Tu D.Y., Ding R., Liang Z.Z., Zhang X.J. Protective effects of Ginkgo biloba leaf extracts on trichloroethylene-induced human keratinocyte cytotoxicity and apoptosis. Skin Pharmacol Physiol. 2005;18:160–169. doi: 10.1159/000085977. [DOI] [PubMed] [Google Scholar]
- Zou Y.P., Lu Y.H., Wei D.Z. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]