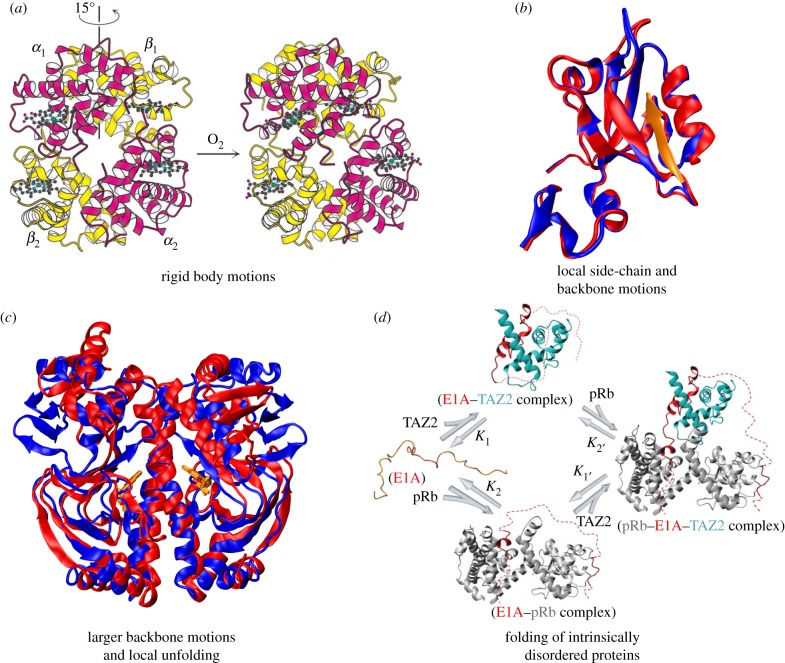
Figure 1.
Allosteric systems representing conformational dynamics of folded structures and large-scale disorder. (a) Haemoglobin is an example of allosteric motion resulting from quaternary structure changes. The binding of oxygen to one haemoglobin subunit induces a 15° rotation of one α/β pair with respect to the other, which raises the affinity of haemoglobin for oxygen, causing the other subunits to also bind oxygen. (b) PDZ domain proteins are an example of allostery without significant structural changes, where ligand binding leads to modulation in distal side-chain motions. The superposition of the structures in the unbound (PDB 1BFE; blue) and bound (PDB 1BE9; red and peptide ligand in orange) states are shown. (c) The allosteric transition in the catabolite activator protein (CAP) upon binding of cyclic adenosine monophosphate (cAMP) is an example of allostery involving larger structural changes and local unfolding. The superposition of apo-CAP (PDB 2WC2; blue) and CAP-cAMP (PDB 1G6N; red) are shown. As CAP is a homodimer, it binds two cAMP molecules, which are highlighted in orange. (d) The binding of the intrinsically disordered protein E1A to the TAZ2 domain of CBP/p300 causes E1A to fold and subsequently bind pRb, yielding a ternary complex. Alternatively, E1A binds first to pRb and then the TAZ2 domain. TAZ2 and pRb do not associate directly, only within ternary complexes formed by binding of both proteins to E1A, which acts as molecular hub involving allosteric regulation. Reproduced with permission from Nature Publishing Group: Ferreon et al. [7]. (Online version in colour.)