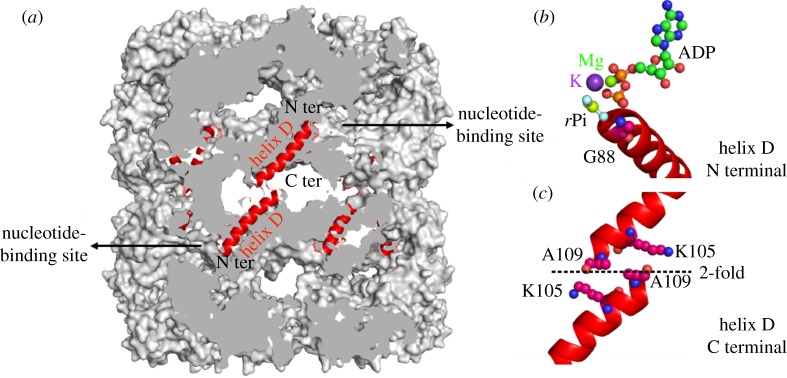
Figure 1.
(a) A sagittal section through apo-GroEL revealing how two nucleotide-binding sites of the different rings communicate allosterically with one another via the helix dipoles of helix D. (b) Owing to the helix dipole, the Gly88 main chain nitrogen atom at the N-terminus of helix D possesses a partial positive charge, suitable for interaction with the βγ-phosphates of ATP. (c) Owing to the helix dipole, the carbonyl oxygen of Ala109 at the C-terminus of helix D possesses a partial negative charge that forms an electrostatic interaction across the twofold axis with the ɛ-amino group of Lys105.