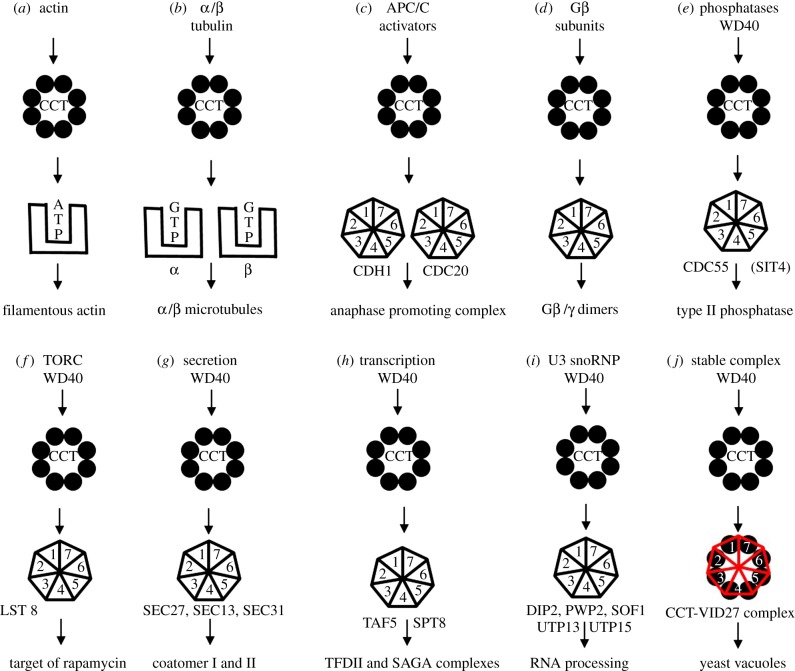
Figure 1.
Cartoons of schemes for CCT action upon its substrates and binding complex formation. (a) Direct folding to native state: actin filaments (F-actin) are composed of a single monomer species (G-actin) and CCT activity yields assembly competent monomers. It is not yet known if ATP loading is concomitant with folding or whether nucleotide-free actin is released into the 2–5 mM ATP pool present in cell cytoplasm and then equilibrates with free ATP. (b) Folding and assembly: tubulin filaments are composed of two monomer species (α-tubulin and β-tubulin) which both bind guanosine triphosphate (GTP). GTP concentration is low in cells (50 µM) and CCT may well play a direct role in GTP loading of β-tubulin. There is strong evidence for post-CCT-dependent folding and assembly step(s), involving several cofactor proteins, which produce filament-assembly competent tubulin monomers. (c) Production of the APC activators: Cdc20p and Cdh1p propeller proteins are CCT-dependent. (d) Assembly of G-protein complexes [18]. (e) Type II phosphatase complex—CCT interaction conserved from yeast to humans. (f) TORC complex: WD40 subunits. (g) Secretory complexes ER and Golgi. (h) Transcription factor and several histone deacetylase complexes interact with CCT [19]. (i) RNA processing. (j) Holding activity of the Saccharomyces cerevisiae Vid27p propeller protein involved in autophagy. (Online version in colour.)