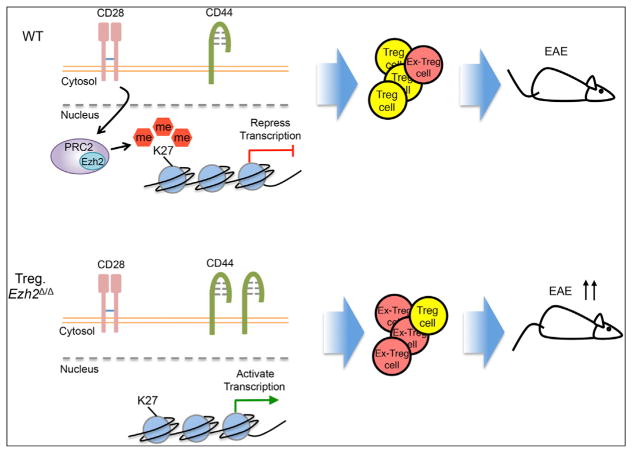
Figure 1. Ezh2 Regulates Function and Stability of Treg Cells.
(Top) Within activated (CD44+) WT Treg cells, CD28 signal enhances the expression of Ezh2, leading to the deposition of genomic H3K27me3 and the repression of transcription of genes that inhibit Treg cell development. Induction of Ezh2 maintains the expression of Foxp3 and stabilizes the identity of Treg cells (shown in yellow). Therefore, the WT Treg cells exert strong suppressive function even in the face of tissue inflammation and during autoimmune reaction in EAE.
(Bottom) In contrast to WT Treg cells, the Ezh2-deficient activated Treg cells fail to promote the repressive gene program for Treg cell development and display a more activated phenotype (CD44+). Therefore, the Ezh2-deficient mice develop more systemic autoimmune reactions with more “Ex-Treg” cells (shown in red), which lose Foxp3 expression and have no suppressive function. The mice show a defect in Treg cell function and massive infiltration of effector and Ex-Tregs in multiple organs. Finally, the absence of Ezh2 within Treg cells leads to the development of more severe EAE compared to WT mice, and the Treg cells lose their identity and function.