Abstract
Background:
Asthma in childhood has a prevalence of 5–10% in Germany and severe asthma accounts for about 5% in this patient group. Positive predictive values for severe asthma are atopy, a positive family history and sensitizations against inhalative allergens. Alternaria is an important inhalative allergen and sensitization is suspected to correlate with severe and lethal asthma. We investigated the prevalence and impact of Alternaria sensitization in paediatric asthma.
Methods:
We reviewed paediatric patients with a diagnosis of low-grade, moderate and severe asthma. Data collection included concomitant atopic diseases, sensitization profiles, family history and prior hospitalization for asthma exacerbation.
Results:
A total of 207 paediatric patients (aged 1–17 years) were included in the study. Overall, 25% had low-grade asthma, 31% moderate and 44% severe asthma and 26% were formerly hospitalized. Alternaria sensitization was the most common in moulds, although without significant correlation with hospitalization and severe asthma. Alternaria sensitization increased with age and was significantly associated with co-sensitization against other moulds, grass pollen and cat epithelia. Allergic rhinitis was significantly correlated with hospitalization, independent of Alternaria sensitization.
Conclusions:
Alternaria sensitization was common and increased with age. No significant correlation was found between asthma degree, hospitalization rates and sensitization profiles. Alternaria sensitization demonstrated no isolated risk factor for severe asthma and hospitalization.
Keywords: Alternaria, asthma, moulds, sensitization
Introduction
As one of the most frequent chronic diseases in childhood, asthma has shown a steadily increasing prevalence throughout the last decades and currently affects up to 10% of all children in industrialized countries [Akinbami et al. 2009; Bacharier et al. 2008]. Allergen exposure, recurrent infections, obesity, tobacco smoke and other inhalative irritants (e.g. volatile compounds or ozone) are associated with inflammatory processes in bronchial tissues. The ensuing presence of a plethora of proinflammatory cells such as mast cells, eosinophils, T-lymphocytes, macrophages or neutrophils causes bronchial constriction, mucosal oedema and mucus production [Jackson et al. 2008; Kabesch et al. 2004]. Remodelling is a feared consequence of the ensuing chronic bronchial inflammation. Therefore, the identification of associated risk factors is of utmost importance, especially to facilitate secondary and tertiary prevention.
Furthermore, sensitization against inhalant allergens such as house dust mites, animal epithelia, pollen and mould allergens are frequently encountered in asthmatic children [Vicencio et al. 2014]. Interestingly, previous studies identified sensitization against the ubiquitous mould Alternaria alternata as an important risk factor for severe asthma associated with increased hospitalization and mortality rates of affected patients [Bush and Prochnau, 2004]. Hence, Alternaria can be regarded as one of the most clinically important moulds worldwide playing a major role in the pathogenesis of allergic rhinoconjunctivitis and asthma [Stark et al. 2005]. Alternaria is a saprophyte thriving on dead plant matter and parasitic on vegetables, grains and within the soil. Although Alternaria belongs to the group of outdoor mould species, indoor burdens are also common due to natural ventilation and indoor flower soil [Koch et al. 2000]. In Europe, climatic peaks of Alternaria exposure are usually reached in July and August as warm temperatures, increased humidity and high wind velocity increase spore release [Bush and Prochnau, 2004].
In adults, Alternaria sensitization is known to be associated with severe asthma and hospitalizations. Thus, antifungal therapy is recommended in sensitized adult patients with uncontrolled asthma [Vicencio et al. 2010]. In contrast, paediatric studies have highlighted a more complex clinical picture, particularly with regard to striking regional differences of Alternaria sensitization rates in asthmatic children (prevalence 1–50%) [Halonen et al. 1997]. Therefore, it was the aim of the current study to depict both the prevalence and the potential clinical relevance of Alternaria sensitization in our cohort of paediatric asthma patients living in rural and urban areas of West Germany.
Materials and methods
Study design
We conducted a retrospective analysis of 207 patients aged 1–17 years with a diagnosis of asthma treated in our department of paediatric pulmonology and allergology at the Rheinisch-Westfälische Technische Hochschule (RWTH) Aachen University Hospital, Germany within a 2-year period (2000–2002). The diagnosis was established by a paediatric pulmonologist according to the Global Initiative for Asthma (GINA) guidelines [GINA, 2002] including history, clinical examination and spirometry. As it was a retrospective analysis with anonymous data collection no ethical approval and no informed consent was necessary.
Data collection
Within the determined time period of 24 months, patient charts were used as primary source of information regarding outpatient visits and hospital stays within our department. Collected data included laboratory results [e.g. fluorescence enzyme immunoassay (FEIA) values for total immunoglobulin E (tIgE) and allergen-specific immunoglobulin E (sIgE), skin prick test (SPT) results] information on concomitant atopic diseases, atopic family history and prior hospitalization due to asthma exacerbations. Classification of asthma severity was performed adhering to the GINA 2002 guideline [GINA, 2002] concerning documented symptoms and lung function results at the first appointment within the time period mentioned above. Only patients with persistent mild, moderate or severe asthma entered the study, whereas children with intermittent asthma and patients with incomplete data sets were excluded from the study. Altogether, 207 patients, aged 1–17 years, were encountered for the study and divided into three age groups. A total of 22/207 patients (10.6%) were younger than 5 years of age and 4 of those (1.9%) were unable to perform spirometry. However, these patients were encountered for analysis and classified, according to GINA 2002, as early infantile asthmatics as all other study parameters correlated with clear atopy and early infant asthma. All other patients had a clear diagnosis with diagnostic work up for asthma.
Pulmonary function tests
Lung function testing was performed with Master Lab software (MasterLab, Inc. CareFusion, Höchberg, Germany) including established reference values for forced spirometry in childhood and adolescence as provided by Zapletal and colleagues [Zapletal et al. 1987]. Precondition was given compliance due to age and the patient’s condition.
Allergy tests
Allergy tests were performed by FEIA analysis (UniCAP® 100, Phadia/Thermofisher, Uppsala, Sweden) as recommended by the manufacturer and comprised a screening panel of inhalant and nutritive allergens (sx1, fx5). Additionally, sIgE levels against Alternaria, Cladosporium herbarum, Aspergillus fumigatus and Penicillium notatum as well as tIgE serum levels were measured and quantified in kU/l.
Only four patients, for whom no FEIA analysis results could be obtained, underwent SPT for the detection of allergen-specific sensitization. SPT was performed according to the guidelines of the European Academy of Allergy and Clinical Immunology using a panel of standardized inhalant allergens (ALK-prick-SQ®, ALK-Scherax, Wedel, Germany) as well as negative and positive controls.
Statistics
Within the predetermined period of time, 207 available data sets were declared as sufficient for analysis. The following parameters were considered: age, sex, asthma severity, concomitant allergic rhinitis and atopic dermatitis, family history of atopy (first-degree relatives), tIgE, sIgE, SPT and history of hospitalization. Data were inserted into an Excel database (Excel 2010, Microsoft) and statistical analysis was performed with Excel and SPSS (SPSS Statistics version 21, IBM). Descriptive analysis was performed (mean, standard deviation, range, median) and cross-tables were established. Correlations were calculated according to the underlying question with Kruskal–Wallis, Mann–Whitney U, Chi-square, Spearman’s rank, Kendall–Tau and Pearson correlation tests. No estimation of minimal sample size for statistical power was performed.
Results
A total of 207 patients aged 1–17 years (mean age 8.56 ± 3.5 years) with a diagnosis of persistent asthma and a complete set of clinical data entered the current study. Overall, 71 (34.3%) were female (mean age 9.01 ± 3.3 years) and 136 (65.7%) were male (mean age 8.32 ± 3.6 years). A total of 48 children (23.2%) also suffered from atopic dermatitis (male versus female = 23.9% versus 22.8%) and 34 (64.7%) patients had allergic rhinitis (male versus female = 63.4% versus 65.4%) while 55 (26.6%) individuals had not been diagnosed with any other atopic diseases. A well-documented family history was available in 196 patients. Of these, 39 patients (19.9%) had a first-degree relative with asthma and 125 patients (63.8%) had a first-degree relative with either allergic rhinitis or atopic dermatitis.
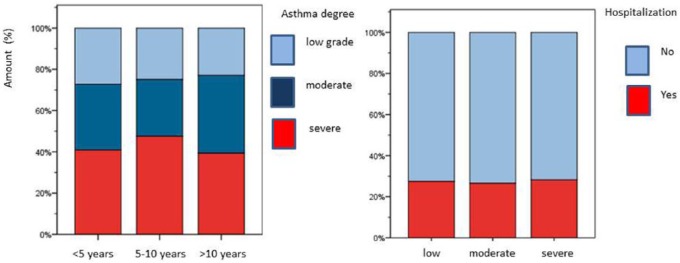
A total of 54 (24.6%) of the recruited patients suffered from mild persistent asthma while moderate and severe asthma was observed in 62 (30.9%) and 92 patients (44.4%) respectively. Dividing our patients into three age-dependent groups, the biggest one was 124 patients (59.9%) aged 5–10 years. Only 22 patients were aged under 5 years (10.6%) and 61 patients were older than 10 years (29.5%). Within these groups asthma severity degrees were comparable without significant age-dependent differences (see Figure 1). Moreover, no significant sex differences could be documented as the rate of female patients only ranged from 31.3–35.9% within the three groups. A total of 57/207 patients (27.5%) had a history of prior hospitalization due to asthma exacerbation (female patients 19.7%, male patients 31.6%). The sex difference however was not significant (Chi-square test, p = 0.07). Moreover, no significant correlation was seen between asthma degree and a history of hospitalization due to acute exacerbations (p = 0.89) as displayed in Figure 1. Likewise, concomitant atopic dermatitis did not demonstrate significant associations with either asthma degree (p = 0.19) or hospitalization rate (p = 0.12). Similarly, allergic rhinitis did not display a significant correlation with asthma degree (p = 0.8) whereas the association of allergic rhinitis and hospitalization was statistically significant (Pearson test, p = 0.03). A positive family history of asthma and atopy showed no correlation with asthma degree (p = 0.73) or hospitalization (p = 0.62).
Figure 1.
Asthma degree within different age groups and prior hospitalization due to asthma exacerbation.
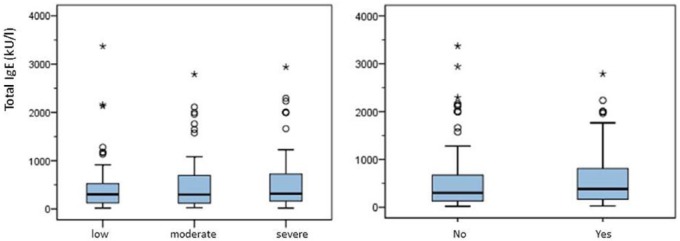
Total IgE (tIgE) levels ranged from 7–3370 kU/l (mean 555 ± 628.42 kU/l, median 307 kU/l). Distribution of median tIgE of low-grade asthma was 300.5 kU/l, of moderate asthma 297.5 kU/l and of severe asthma 317.5 kU/l. A statistically significant correlation was seen between increasing age and levels of tIgE (Spearman’s rank correlation, p = 0.02), whereas no significant association with various other demographic and clinical factors could be established. Female patients had slightly higher tIgE levels (mean 613 kU/l versus 524 kU/l), however without significance (Mann–Whitney U-test, p = 0.6). Levels of tIgE showed no significant correlation with asthma degree (Kruskal–Wallis, p = 0.73) and prior hospitalization (see Figure 2) due to asthma exacerbation (Mann–Whitney U-test, p = 0.31). Details are displayed in a cross-table (see Table 1).
Figure 2.
Total IgE levels in correlation with asthma degree (left) and hospitalization (right).
IgE, immunoglobulin E.
Table 1.
Subject data of asthma degree, hospitalization and tIgE (mean ± SD) in context with sex.
| Asthma degree | |||||
|---|---|---|---|---|---|
| Sex |
Overall | ||||
| Female | Male | ||||
| Asthma degree | Mild | Number | 18 | 33 | 51 |
| % within sex | 25.4% | 24.3% | 24.6% | ||
| Moderate | Number | 20 | 44 | 64 | |
| % within sex | 28.2% | 32.4% | 30.9% | ||
| Severe | Number | 33 | 59 | 92 | |
| % within sex | 46.5% | 43.4% | 44.4% | ||
| Entire | Number | 71 | 136 | 207 | |
| % within sex | 100.0% | 100.0% | 100.0% | ||
| Hospitalization | |||||
| Sex |
Entire | ||||
| Female | Male | ||||
| Hospitalization | No | Number | 57 | 93 | 150 |
| % within sex | 80.3% | 68.4% | 72.5% | ||
| Yes | Number | 14 | 43 | 57 | |
| % within sex | 19.7% | 31.6% | 27.5% | ||
| Entire | Number | 71 | 136 | 207 | |
| % within sex | 100.0% | 100.0% | 100.0% | ||
| total IgE levels | |||||
| Sex |
Entire | ||||
| Female | Male | ||||
| tIgE (kU/l) | Mean | 613 | 524 | 555 | |
| SD | 705 | 584 | 628 | ||
IgE, immunoglobulin E; tIgE, total immunoglobulin E.
Allergen-specific sensitization, as determined by FEIA analysis in 203 patients (98.1%), was foremost against grass pollen (70.2%), followed by Dermatophagoides pteronyssinus (70%), Dermatophagoides farinae (69.4%), dog epithelia (44.3%), cat epithelia (42.6%) and tree pollen (40.3%). Alternaria alternata was the most frequently recognized fungal antigen (35/203 patients, 17.2%) followed by Aspergillus fumigatus (23/203 patients, 11.3%), Cladosporium herbarum (22/203 patients, 10.8%) and Penicillium notatum (18/203 patients, 8.9%).
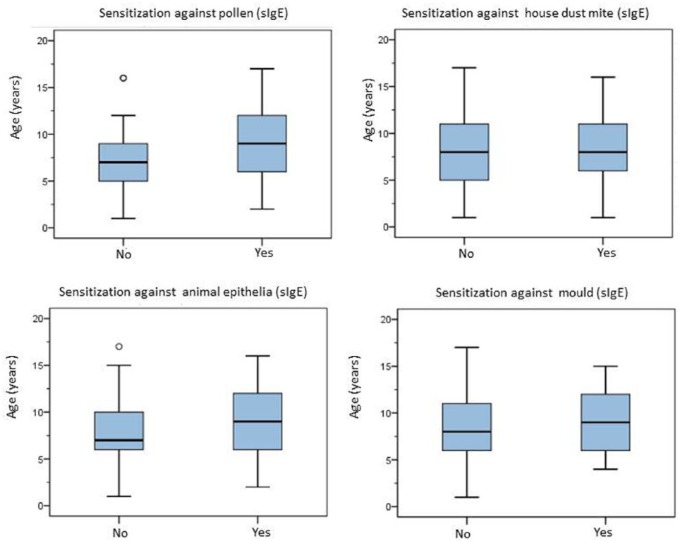
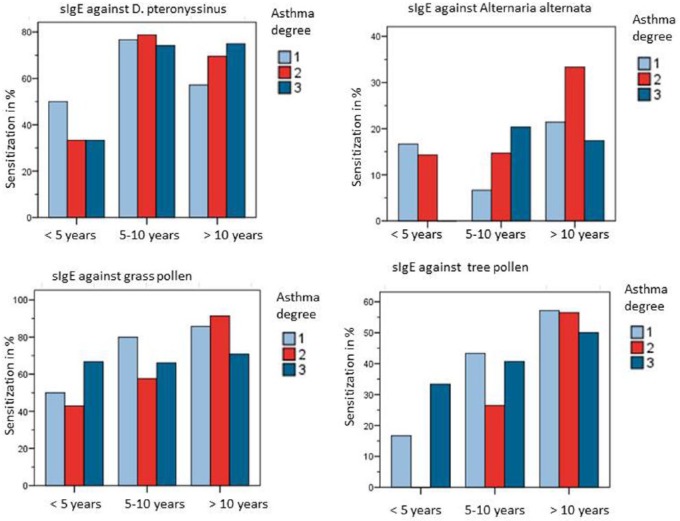
A distribution of age in connection with specific sensitization is displayed in Figure 3. Patient age was significantly correlated with the number of allergen-specific sensitizations (Spearman’s correlation coefficient: p < 0.001) whereas no correlation could be established between asthma severity, age and the frequency of sensitization against pollen (p = 0.128), house dust mite (p = 0.776), animal epithelia (p = 0.675) or mould (p = 0.483) as shown in Figure 4. Only within the group of 5–10-year-old patients Alternaria sensitization was correlated with an increase of asthma degree, however again without statistical significance (p = 0.097, each Chi-square testing). Accordingly, patients with moderate or severe asthma were not more frequently sensitized (sIgE) to inhalant allergens as compared with patients with low-grade asthma. Concerning allergen-specific sensitization (sIgE) and hospitalization, no significant correlation was seen in neither Alternaria sensitization (p = 0.43), nor tree pollen (p = 0.2), grass pollen (p = 0.09), Dermatophagoides pteronyssinus (p = 0.439), Dermatophagoides farinae (p = 0.29), cat epithelia (p = 0.06) or dog epithelia (p = 0.14).
Figure 3.
Allergen-specific sensitization (sIgE) towards pollen, house dust mite, animal epithelia and mould depending on age.
sIgE, allergen-specific immunoglobulin E.
Figure 4.
Sensitization (sIgE) against D. pteronyssinus, Alternaria alternata, grass and tree pollen and asthma degree within different age groups. Alternaria sensitization in group ‘5–10 years’ is correlated with an increase of asthma degree, however without statistical significance (p = 0.097, Chi-square test).
sIgE, allergen-specific immunoglobulin E.
Note: Patients were aged <5 years with a diagnosis of early infant asthma.
We observed significant co-sensitization (sIgE) between Alternaria and other moulds, in particular Penicillium notatum (Kendall–Tau, p < 0.01), Cladosporium herbarum (p < 0.01) and Aspergillus fumigatus (p < 0.01). Moreover, significant co-sensitization (sIgE) was documented between Alternaria and tree pollen (Kendall–Tau, p < 0.01), grass pollen (p < 0.01) and cat epithelia (p = 0.04). Alternaria sensitization (sIgE) increased with age with a proportion of 9.1% in children below 5 years of age, rising to 15.4% in 5–10-year-old children to 24.1% in children and adolescents above 10 years of age.
However, a correlation between Alternaria sensitization and asthma degree was not discovered in each age group. Similar findings were observed in sensitization to Aspergillus, Penicillium notatum and Cladosporium herbarum (see Table 2). Patients with hospitalization had more frequent sensitization to Alternaria (27%) than nonhospitalized patients (73%), nevertheless without statistical significance (Chi-square, p = 0.359). Total IgE levels were increased in patients with allergen-specific sensitization against Alternaria (717 kU/l versus 520 kU/l), however without significance (Mann–Whitney U-test, p = 0.282).
Table 2.
Detection of specific IgE against moulds (% of each group) in correlation with an increase in the degree of asthma within different age groups (p-values by Chi-square test).
| Allergen | Group <5 years (n = 22) | Group 5–10 years (n = 123) | Group >10 years (n = 58) |
|---|---|---|---|
| Alternaria alternata | 9.1%, p = 0.403 | 15.4%, p = 0.097 | 24.1%, p = 0.701 |
| Aspergillus fumigatus | 4.5%, p = 1 | 11.4%, p = 0.56 | 13.8%, p = 0.81 |
| Penicillium notatum | 4.5%, p = 1 | 8.9%, p = 0.251 | 10.3%, p = 0.25 |
| Cladosporium herbarum | 4.5%, p = 1 | 8.9%, p = 0.123 | 17.2%, p = 0.38 |
IgE, immunoglobulin E.
Discussion
In 1873 Charles Blackley first described the potential role of moulds in chronic respiratory diseases [Blackley, 1873]. In 1928 Hansen first characterized mould-associated asthma [Hansen, 1928]. Since the 1980s fungal sensitization entered the limelight again as atopic diseases increased significantly. Since then, many studies have addressed this question. Meanwhile ‘severe asthma with fungal sensitization’ is a distinct phenotype of asthma [Denning et al. 2006]. An increase in hospitalization due to asthma exacerbations is documented in several studies during the main spore count in sticky and warm weather in summer and early autumn [O’Driscoll et al. 2005]. In addition, the focus was guided to allergen-specific sensitization against Alternaria and potentially lethal asthma exacerbations or severe degrees of asthma [Neukirch et al. 1999]. Alternaria exposure occurs mainly outdoors, however indoor concentrations seem to fluctuate concerning the outdoor spore counts [Koch et al. 2000].
In our cohort of paediatric patients with persistent mild, moderate and severe asthma, Alternaria was the most frequent sensitizing mould allergen (17.2%) whereas grass pollen, house dust mite and animal epithelia were the leading inhalant allergens with documented sensitization. The distribution of sensitization profiles in our cohort is equivalent to those reported in other studies in Northern and mid-Europe [O’Driscoll et al. 2005; Niemeijer and de Monchy, 1992]. Although sensitization against Alternaria was common in our cohort, a correlation between asthma degree and hospitalization could not be established. Likewise, other specific sensitizations against inhalant allergens were not correlated with asthma severity or hospitalization rates. Study results concerning allergen-specific sensitization and asthma degree or hospitalization rates are conflicting and range from no correlation [Gergen et al. 2002] to a highly significant correlation, especially in cases of sensitization to Alternaria or pollen allergens [Galan et al. 2010; Knutsen et al. 2010]. A possible explanation might be climatic conditions affecting allergen exposure. Different investigations have elaborated the context of predominant allergens and sensitization patterns in children with asthma. In Germany, Alternaria is a leading mould allergen, followed by Aspergillus, Cladosporium and Penicillium [Fangmeyer and Zorn, 2007]. Of note, it is well known that sensitization increases with exposure in patients with atopic susceptibility. As there is a seasonal increase in spore counts in July and August in our German cohort; seasonal increase of asthma degree and hospitalization seems to be argumentative in sensitized patients [Kilic et al. 2010]. In our cohort no association was found between asthma degree and risk of hospitalization. Furthermore, no significant correlation between asthma degree or hospitalization and Alternaria sensitization could be detected. As expected, an increase in tIgE levels in older patients was not accompanied by an increase in the degree of asthma.
A possible limitation of this retrospective study design is that mild exacerbations without the need for hospitalization were not identified. Hospitalization was not systematically documented in the context of seasonal or climatic conditions; moreover no information about repeated hospitalization was provided. Also, prior hospitalization in the temporal context to the declaration of severe asthma was not possible. Hence, no statement concerning the influence of Alternaria exposure in sensitized patients is possible. In other studies, exposure and sensitization to perennial indoor allergens in the context of severe asthma has been discussed [Illi et al. 2006]. Even though the prevalence of allergen-specific sensitization to house dust mite and animal epithelia in our collective was high, no correlation was seen concerning asthma degree or hospitalization. The only significant correlation was observed between asthmatic patients with concomitant allergic rhinitis and the need for hospitalization. A possible reason might be the additive effect of inflammation of the upper and lower airways and a simultaneous and protracted burden of allergen exposure. As our patients were recruited in a tertiary referral centre, a possible selection bias also has to be kept in mind. In accordance, patients with intermittent or low-grade asthma were under-represented or missing.
Age distribution seems to play an important role. Nolles and colleagues published similar results with predominant sensitization to Alternaria and Cladosporium in patients aged 6–10 years, followed by a decrease of the prevalence in older patients [Nolles et al. 2001]. In contrast, a sensitization against house dust mite was primarily documented in patients aged 4 years followed by stabilization of sensitization levels [Niemeijer and de Monchy, 1992]. Similar results were observed in our cohort with increasing sensitization against Alternaria above 10 years of age and against house dust mite within the age group of 5–10-year-old patients, then remaining constant. The only patient group displaying a correlation between asthma degree and sensitization against Alternaria, Aspergillus and Penicillium were the patients of 5–10 years of age, although not statistically significant. It has to be kept in mind that, especially in the group of preschool patients, other causes for exacerbations (e.g. viral or bacterial infection) might be responsible for hospitalization. Unfortunately, due to the retrospective study design no differentiation of reasons for hospitalization was possible.
For statistical power at least 0.8, a minimal sample size should have been evaluated. This might have led to statistical errors within this study. Moreover, differing numbers of patients within the three age groups may have furthermore influenced the observed effects. One important aspect is that we only discuss allergen-specific sensitization, detected by FEIA analysis or, in a minor group of our patients, by SPTs whereas no nasal or conjunctival provocation tests were performed to differentiate sensitization from true allergy. Moreover, it remains to be elucidated if component-resolved sIgE detection would have improved sensitivity and specificity of FEIA assays with Alt a 1 as the major allergen of Alternaria and thus excluding potential cross-reactions to other moulds or pollen [Gergen et al. 2002; Chruszcz et al. 2012].
Statistical analysis focused on the detection of sIgE and not SPTs due to the low number of patients. The described age-dependent association of the 5–10-year-old patients and Alternaria sensitization was established by sIgE detection. Different studies exist identifying an increase of tIgE as a potential risk factor for severe asthma and hospitalizations [Halonen et al. 1997]. In our cohort we also observed rising tIgE levels in patients with higher degrees of asthma or a prior history of hospitalization. However these associations did not reach statistical significance, which is in accordance with the findings of other research groups [Frith et al. 2011]. Previous studies have addressed the subject of nonallergic airway inflammation due to fungal antigens (e.g. by release of proinflammatory cytokines caused by fungal proteases) and epithelial damage to the respiratory epithelia [Kauffmann et al. 2000]. Moreover, colonization of the respiratory epithelia with Alternaria and other moulds might be risk factors for exacerbations.
The treatment of mould-sensitized, asthmatic children with antifungal drugs such as itraconazole is still a matter of debate. In general, asthmatic children do not develop disturbances of mucociliary clearance. Additionally, antifungal drugs may cause side effects, interactions with other drugs and there may be problems finding the correct treatment dosage [Lehmann et al. 2014]. In conclusion, sensitization against moulds and asthma in childhood alone is no reason for antifungal treatment. This is corroborated by controversial study results concerning the real risk of Alternaria and other mould-sensitization in asthmatic children and adolescents [Knutsen et al. 2010].
A change of paradigm concerning degrees of asthma was published by the GINA in 2006 (GINA, 2006). Since then, asthma is no longer classified as intermittent, low, moderate or severe, but is currently termed controlled, intermittent controlled or uncontrolled asthma. As we could not retrospectively apply this classification to our patient cohort, this may hamper the transferability of our data to other patient groups subdivided according to more recent GINA criteria. Nevertheless, even nowadays asthma therapists make use of asthma degree classifications for a first assessment of asthma therapy. Overall, 10.6% of our study patients were below 5 years old and 1.9% did not have spirometry, which would have been necessary for the GINA 2002 classification. An investigation of patients above 5 years only would have been better as reliable spirometry results could have been taken into account.
We feel that the results of our investigation are worthwhile being considered as they further characterize the clinical role of Alternaria sensitization in a sizeable population of paediatric asthma patients. Further studies should be performed to detect changes of sensitization profiles and the relevance of sensitization against Alternaria and other moulds at present age.
Conclusion
In conclusion, this study revealed that sensitization to Alternaria alternata is common in German children with different degrees of asthma severity. However, no significant association of IgE-mediated sensitization against this mould with either asthma degree or risk of asthma-related hospitalizations could be established. This finding might further underline that antifungal therapy is not warranted in Alternaria-sensitized asthmatic children a priori. Rather, a detailed history and nasal provocation testing should be performed to differentiate clinically-irrelevant sensitization from genuine allergy.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Sylvia Lehmann, Department of Pediatric Pulmonology and Allergology, University Hospital RWTH Aachen, Pauwelsstrasse 30, 52074 Aachen, Germany.
Anja Sprünken, University Hospital RWTH Aachen, Germany.
Norbert Wagner, University Hospital RWTH Aachen, Germany.
Klaus Tenbrock, University Hospital RWTH Aachen, Germany.
Hagen Ott, Children’s Hospital Auf der Bult, Hannover, Germany.
References
- Akinbami L., Moorman J., Garbe P. (2009) Status of childhood asthma in the United States, 1980–2007. Pediatrics 123(Suppl. 3): 131–145. [DOI] [PubMed] [Google Scholar]
- Bacharier L., Boner A., Carlsen K., Eigenmann P., Frischer T., Götz M., et al. (2008) Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy 63: 5–34. [DOI] [PubMed] [Google Scholar]
- Blackley C. (1873) Experimental Researches on the Cause and Nature of Cararrhus Aestivus. London: Baillière, Tindall and Cox; Available at : https://catalog.hathitrust.org/Record/012406867. [Google Scholar]
- Bush R., Prochnau J. (2004) Alternaria-induced asthma. J Allergy Clin Immunol 113: 227–234. [DOI] [PubMed] [Google Scholar]
- Chruszcz M., Chapman M., Osinski T., Solberg R., Demas M., Porebski P., et al. (2012) Alternaria alternate allergen Alt a 1: a unique beta-barrel protein dimer found exclusively in fungi. J Allergy Clin Immunol 130: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning D., O’Driscoll B., Hogaboam C., Bowyer P., Niven R. (2006) The link between fungi and severe asthma: a summary of the evidence. Eur Respir J 27: 615–626. [DOI] [PubMed] [Google Scholar]
- Fangmeyer T., Zorn C. (2007) Verteilung der Konzentration Luftgetragener, Keimfähiger Schimmelpilzsporen in Innenraum und Außenluft bei Nachweis über Filtration. ÄGÖF, Hrsg. Umwelt, Gebäude & Gesundheit. Springe Eldagsen: 264–275. [Google Scholar]
- Frith J., Fleming L., Bossley C., Ullmann N., Bush A. (2011) The complexities of defining atopy in severe childhood asthma. Clin Exp Allergy 41: 948–953. [DOI] [PubMed] [Google Scholar]
- Galan I., Prieto A., Rubio M., Herrero T., Cervigón P., Cantero J., et al. (2010) Association between airborne pollen and epidemic asthma in Madrid, Spain: a case-control study. Thorax 65: 398–402. [DOI] [PubMed] [Google Scholar]
- Gergen P., Mitchell H., Lynn H. (2002) Understanding the seasonal pattern of childhood asthma: results from the National Cooperative Inner-City Asthma Study (NCICAS). J Pediatr 141: 631–636. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) (2002) GINA Science Committee. Global Strategy for Asthma Management and Prevention. Available at: www.ginasthma.org.
- Global Initiative for Asthma (GINA) (2006) GINA Science Committee. Global Strategy for Asthma Management and Prevention. Available at: www.ginasthma.org.
- Halonen M., Stern D., Wright A., Taussig L., Martinez F. (1997) Alternaria as a major allergen for asthma in children raised in a desert environment. Am J Respir Crit Care Med 155: 1356–1361. [DOI] [PubMed] [Google Scholar]
- Hansen K. (1928) Über Schimmelpilz-Asthma. Verhandl Deutsch Gesellsch Inn Med 40: 204. [Google Scholar]
- Illi S., Von Mutius E., Laur S., Niggemann B., Grüber C., Wahn U. (2006) Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet 368: 763–770. [DOI] [PubMed] [Google Scholar]
- Jackson D., Gangnon R., Evans M., Roberg K., Anderson E., Pappas T., et al. (2008) Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med 178: 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabesch M., Hoefler C., Carr D., Leupold W., Weiland S., von Mutius E. (2004) Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax 59: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffmann H., Tomee J., van de Riet M., Timmerman A., Borger P. (2000) Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol 105: 1185–1193. [DOI] [PubMed] [Google Scholar]
- Kilic M., Ufuk Altintas D., Yilmaz M., Güneşer Kendirli S., Bingöl Karakoc G., Taskin E., et al. (2010) The effects of meteorological factors and Alternaria spore concentrations on children sensitized to Alternaria. Allergol Immunopathol (Madr) 38: 122–128. [DOI] [PubMed] [Google Scholar]
- Knutsen A., Vijay H., Kumar V., Kariuki B., Santiago L., Graff R., et al. (2010) Mold-sensitivity in children with moderate-severe asthma is associated with HLA-DR and HLA-DQ. Allergy 65: 1367–1375. [DOI] [PubMed] [Google Scholar]
- Koch A., Heilemann K., Bischof W., Heinrich J., Wichmann H. (2000) Indoor viable mold spores – a comparison between two cities, Erfurt (eastern Germany) and Hamburg (western Germany). Allergy 55: 176–180. [DOI] [PubMed] [Google Scholar]
- Lehmann S., Pfannenstiel C., Friedrichs F., Kröger K., Wagner N., Tenbrock K. (2014) Omalizumab: a new treatment option for allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Ther Adv Respir Dis 8: 141–149. [DOI] [PubMed] [Google Scholar]
- Neukirch C., Henry C., Leynaert B., Liard R., Bousquet J., Neukirch F. (1999) Is sensitization to Alternaria alternate a risk factor for severe asthma? A population-based study. J Allergy Clin Immunol 103: 709–711. [DOI] [PubMed] [Google Scholar]
- Niemeijer N., de Monchy J. (1992) Age-dependency of sensitization to aero-allergens in asthmatics. Allergy 47: 431–435. [DOI] [PubMed] [Google Scholar]
- Nolles G., Hoekstra M., Schouten J., Gerritsen J., Kauffman H. (2001) Prevalence of immunoglobulin E for fungi in atopic children. Clin Exp Allergy 31: 1564–1570. [DOI] [PubMed] [Google Scholar]
- O’Driscoll B., Hopkinson L., Denning D. (2005) Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark P., Celedon J., Chew G., Ryan L., Burge H., Muilenberg M., et al. (2005) Fungal levels in the home and allergic rhinitis by 5 years of age. Environ Health Perspec 113: 1405–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicencio A., Muzumdar H., Tsirilakis K., Kessel A., Nandalike K., Goldman D. (2010) Severe asthma with fungal sensitization in a child: response to itraconazole therapy. Pediatrics 125: 1255–1258. [DOI] [PubMed] [Google Scholar]
- Vicencio A., Santiago M., Tsirilakis K., Stone A., Worgall S., Foley E., et al. (2014) Fungal sensitization in childhood persistent asthma is associated with disease severity. Pediatr Pulmonol 49: 8–14. [DOI] [PubMed] [Google Scholar]
- Zapletal A., Samanek M., Paul T. (1987) Lung function in children and adolescents. Prog Respir Res 22: 1–8. [Google Scholar]