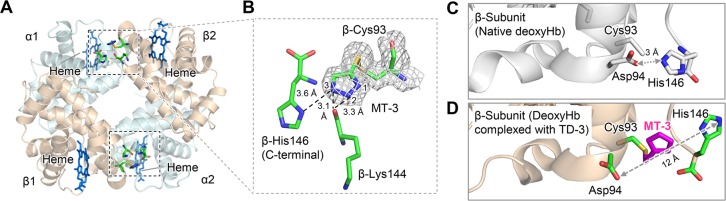
Figure 5.
Crystal structure of deoxyHbA in a complex with TD-3. MT-3 formed a disulfide bond with the thiol of deoxyHbA β-Cys93. (A) The binding sites of MT-3 in deoxyHbA. Hb α and β subunits are shown in pale blue and brown, respectively. The locations of MT-3, β-Cys93, β-Lys144, and β-His146 are shown as sticks within the dashed rectangular areas. (B) The electron density of MT-3 and β-Cys93 indicated by the 2Fo-Fc map (gray mesh, contoured at 1.0σ). (C) β-subunit of the crystal structure of native deoxyHbA (PDB ID: 2DN2). β-Asp94 and β-His146 formed the characteristic salt-bridge to stabilize the T-state Hb. (D) β-subunit of the crystal structure of deoxyHbA in a complex with TD-3. The binding of MT-3 at the thiol of β-Cys93 disrupted the salt-bridge interaction between β-Asp94 and β-His146.