Abstract
Objective:
PAX6 haploinsufficiency (+/−) can occur due to mutations involving only PAX6 in patients with isolated aniridia or as contiguous gene deletions in patients with Wilms tumor, aniridia, genitourinary anomalies, and range of developmental and intellectual disabilities syndrome. Given the role of PAX6 in pineal development and circadian regulation, adolescents with PAX6+/− may experience sleep–wake disturbances. The purpose of this observational study was to explore sleep-related phenotypes in adolescents with PAX6+/−.
Methods:
This study compared sleep phenotypes of nine subjects with PAX6+/− (aged 10–19 years) with previously published data on healthy adolescents (n = 25, aged 10–18 years). Subjects completed the Cleveland Adolescent Sleepiness Questionnaire (CASQ), Patient Reported Outcomes Measurement Information System (PROMIS) Sleep Disturbance (v. 1.0; 8a), and PROMIS Sleep-Related Impairment (v. 1.0; 8b) Questionnaires and wore actigraphs for seven nights to record sleep patterns.
Results:
Total CASQ, PROMIS sleep-related impairment, and PROMIS sleep disturbance scores were not statistically different between the groups (ps > .15). Actigraph data for lights off to sleep-onset time were found to be significantly higher in subjects with PAX6+/− versus the healthy comparison group (adjusted mean [95% confidence interval]: 20.1 min [8.1, 49.8] vs. 6.2 min [3.7, 10.4], respectively, p = .04).
Conclusion:
Both adolescents with PAX6+/− and the healthy comparison group on average slept less than 8 hr/night, and overall sleep deprivation in adolescents may have masked differences between groups. This study used rare genetic disorders with biological vulnerability to sleep problems as a genotype–phenotype model. Knowledge of sleep-related phenotypes will assist in designing studies to manage sleep-related symptoms in adolescents.
Keywords: sleep, adolescence, PAX6, CASQ, PROMIS, actigraphy
PAX6 haploinsufficiency (+/−) can occur due to mutations involving only PAX6 in patients with isolated aniridia, a rare developmental eye disorder, or as part of 11p13 contiguous gene deletions of variable size that involve PAX6 and several neighboring genes in patients with Wilms tumor, aniridia, genitourinary anomalies, range of developmental and intellectual disabilities (WAGR) syndrome (Fischbach, Trout, Lewis, Luis, & Sika, 2005; Lee, Khan, & O’Keefe, 2008). In addition to its role in eye development, PAX6 appears to be important for the development of the pineal gland, the primary source of the circadian-regulating hormone melatonin (N-acetyl-5-methoxytryptamine; Abouzeid et al., 2009; Estivill-Torrus, Vitalis, Fernandez-Llebrez, & Price, 2001; Mitchell et al., 2003; Rath et al., 2009; Walther & Gruss, 1991).
We previously reported significantly reduced pineal volume, reduced melatonin secretion, and greater parent report of sleep disturbance in subjects with PAX6+/− versus healthy controls (Hanish, Butman, Thomas, Yao, & Han, 2016). Our study was the first to explore the functional consequences of pineal hypoplasia in subjects with PAX6+/−, and our findings support the hypothesis that PAX6 plays an important role in human pineal development and sleep regulation (Hanish et al., 2016). Subjects with WAGR syndrome and isolated aniridia can serve as genotype–phenotype models for studying human PAX6+/− sleep-related phenotypes, which is essential for establishing effective sleep management in these patients. Parent report of sleep may underestimate children’s sleep problems, and as children get older, parents may be unaware of their child’s increased sleep-onset delay and frequency and duration of any night awakenings (Baker, Richdale, Short, & Gradisar, 2013; Goldman, Richdale, Clemons, & Malow, 2012; J. A. Owens, Spirito, McGuinn, & Nobile, 2000). In the extant medical literature, self-reported daytime sleepiness, sleep disturbance, sleep-related impairment, and sleep patterns have not been described in subjects with PAX6+/−.
Although PAX6+/− is rare and research on the role of PAX6 in circadian regulation limited, a broader area of interest is investigations of the irregular patterns of sleep–wake rhythm and abnormalities in melatonin physiology frequently observed in children and adolescents with neurodevelopmental disorders (NDDs) such as autism spectrum disorder and attention deficit hyperactivity disorder (Novakova et al., 2011; Rossignol & Frye, 2014). Sleep disturbances in children and adolescents with NDDs include long sleep-onset latency, frequent awakenings, bedtime resistance, poor sleep efficiency, and less than optimal total sleep time (TST; Lunsford-Avery, Krystal, & Kollins, 2016; Sivertsen, Posserud, Gillberg, Lundervold, & Hysing, 2012). Sleep-related disturbances are a clinically important issue in children and adolescents with NDDs because sleep problems can exacerbate and worsen repetitive and stereotypic behavior, inattention, and hyperactivity as well as interfere with learning and cognition (Cohen, Conduit, Lockley, Rajaratnam, & Cornish, 2014; Hirata et al., 2015; Malow, McGrew, Harvey, Henderson, & Stone, 2006; Taylor, Schreck, & Mulick, 2012; Wiggs & Stores, 1996). Sleep problems in children and adolescents with NDDs can also adversely affect parental sleep and family functioning (Goldman, Wang, & Fawkes, 2014; Jan et al., 2008; Lopez-Wagner, Hoffman, Sweeney, Hodge, & Gilliam, 2008; Meltzer, 2008). Ascertaining sleep-related phenotypes in patients with WAGR and isolated aniridia is necessary as sleep problems may go unrecognized and undertreated, which may exacerbate existing conditions.
According to the 2016 NINR Strategic Plan: Advancing Science, Improving Lives, nurse scientists are well suited for patient-centered biobehavioral research including symptom science (e.g., sleep disturbance). As the National Institute of Nursing Research (2016) plan explains, symptom management research improves our understanding of the genomic and biological mechanisms associated with symptoms while at the same time increasing our knowledge of the behavioral aspects of symptoms to reduce their disabling effects and improve patient health outcomes and quality of life. Although WAGR syndrome and isolated aniridia are very rare genetic disorders, to our knowledge, they are the only reported disorders associated with congenital pineal hypoplasia.
Evidence suggests that sleep disturbances in children and adolescents are likely multifactorial, attributable to intrinsic pathophysiological factors, physical and psychiatric comorbidities, and pharmacological and parental influences (Stores, 2016). Insufficient sleep among adolescents is common and grows progressively worse over the course of adolescence (J. Owens, Adolescent Sleep Working Group, & Committee on Adolescence, 2014). Our prior research suggests that adolescents with PAX6+/− may be particularly vulnerable to adverse sleep-related phenotypes due to the role of PAX6 in pineal development and subsequent melatonin deficiency (Hanish et al., 2016). In that study, we described the sleep-related phenotypes in this population to provide insights on the functional consequences of melatonin deficiency with applicability to more common conditions such as NDDs. In another prior study, we examined the utility of self-reported measures for assessing sleep disturbance and sleep-related impairment in healthy adolescents (Hanish, Lin-Dyken, & Han, 2017). The purpose of the present observational study was to further explore sleep-related phenotypes in adolescents with PAX6+/−. We hypothesized that PAX6+/− would be associated with greater daytime sleepiness, sleep disturbance, and sleep-related impairment as well as increased sleep-onset latency compared to our previously reported healthy comparison group.
Material and Method
Study Population
Participants included adolescents with WAGR syndrome or isolated aniridia diagnosed by clinical examination and prior genetic testing confirming a PAX6 mutation or deletion. Eligible adolescents met the following criteria: (1) 10–20 years old, (2) able to perceive light (lack of light perception could lead to free-running circadian rhythm), (3) not taking any sleep aids, including melatonin, for at least 7 days, and (4) IQ > 70 or, if IQ test results were not available, parent-reported functional capacity to complete study questionnaires. We recruited 12 adolescents with PAX6+/− who were eligible for the study from participants in an existing protocol and responses to recruitment fliers sent to aniridia/WAGR organizations. Of these, three were unable to stop melatonin for the purposes of this study; thus, nine adolescents met inclusion criteria and were included in the sample. The healthy comparison subjects had no chronic medical conditions, were not taking any chronic medications, and were previously described (Hanish et al., 2017). We enrolled both adolescents with PAX6+/− and those in the healthy comparison group April to May 2014 and September to October 2014 and collected data during a typical school week. The institutional review board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, approved the study. We obtained written informed consent from parents of adolescents and assent from the adolescents. All participants received US$50 compensation for completion of the 7-day actigraphy recording and US$10 for completion of study questionnaires.
Measurements
Cleveland Adolescent Sleepiness Questionnaire (CASQ)
The CASQ is a 16-item measure of daytime sleepiness for adolescents 11–17 years of age. The CASQ is a self-report measure, with higher scores indicative of increased daytime sleepiness (Spilsbury, Drotar, Rosen, & Redline, 2007). The CASQ has demonstrated good reliability (α = .89), correlations (r = .66–.75) with two other measures of daytime sleepiness (Sleep Habits School Survey and Pediatric Daytime Sleepiness Scale), and construct validity in community samples (Spilsbury et al., 2007). A previous study reported normative sample CASQ scores (mean ± standard deviation: 31.2 ± 9.4) as well as CASQ scores for primary snorers (35.0 ± 12.3) and sleep apnea (37.7 ± 11.5; Spilsbury et al., 2007).
Patient Reported Outcomes Measurement Information System (PROMIS) sleep disturbance and sleep-related impairment
We measured sleep disturbance and sleep-related impairment using the adult PROMIS 8-item short forms, as no pediatric sleep measures were available in any of the sleep domains (Health Measures, 2017). The PROMIS Sleep Disturbance Questionnaire (v. 1.0; 8a) is a self-report measure of sleep quality, depth, and restoration within the past 7 days. We also used the PROMIS Sleep-Related Impairment Questionnaire (v. 1.0; 8b), a self-report measure of alertness, sleepiness, tiredness, and functional impairments during waking hours associated with sleep problems within the past 7 days. Both PROMIS sleep measures use a 5-point Likert-type scale with raw scores converted to a standardized T-score. Conversion tables are published on the Health Measures website (http://www.healthmeasures.net), with higher scores indicating greater sleep–wake disturbances. The Pittsburgh Sleep Quality Index, one of the adult legacy sleep measures, correlates significantly with the sleep disturbance bank (r = .85) and the sleep-related impairment bank (r = .70; Cella et al., 2010; Health Measures, 2017). The adult PROMIS sleep measures have demonstrated adequate content validity in adolescents, aged 12–18 years (van Kooten, Terwee, Kaspers, & van Litsenburg, 2016). We have also published preliminary validation of the adult PROMIS sleep disturbance and sleep-related impairment measures in healthy adolescents, aged 10–18 years (Hanish et al., 2017). We found significant correlations between the PROMIS sleep measures and the CASQ (all Spearman’s r > .7). TST determined by actigraphy was negatively correlated with both PROMIS sleep disturbance (p = .02) and sleep-related impairment (p = .02), indicating that higher TST measured by actigraphy was correlated with less sleep disturbance and sleep-related impairment.
Actigraphy
We used the Actiwatch Spectrum device (Philips Respironics, Bend, OR) as an indirect, objective measure of sleep–wake patterns. Subjects wore the device on their nondominant wrist in the home setting for 7 consecutive days and nights during a typical school week to provide an estimate of sleep based on movement. The actigraph was recorded in 30-s epochs and was set at a medium sensitivity level for scoring sleep and wake time. We uploaded actigraphy data to a computer, and an automated algorithm provided an overall average value for the 7-day period including total bedtime, total wake time, TST, sleep efficiency, and sleep-onset latency (Respironics Actiware 6, Philips Respironics, Bend, OR). A custom interval was generated to calculate the time from lights off (using the actigraph sensor with automated light thresholds) to sleep onset. In addition, sleep variables were split into weekday school nights (Sunday–Thursday) and weekend nights (Friday and Saturday), and average values were calculated. Participants completed a 7-day sleep/wake log to record the approximate time of sleep and wake (Philips Respironics, Bend, OR). Procedures were guided by a previous report aimed at standardizing publishing of actigraphy results (Berger et al., 2008).
Statistical Analyses
We performed statistical analyses using SPSS Version 23.0 (IBM Corp., Armonk, NY). Skewness, kurtosis, and Kolmogorov–Smirnov test for normality were assessed. Independent samples t tests, Mann–Whitney U tests, and Fisher’s exact tests were used to compare unadjusted differences in demographic and clinical characteristics between adolescents with PAX6+/− and the previously reported healthy comparison group. Skewed data were normalized by log transformation, so that parametric analyses of covariance could be used to assess differences in actigraphy variables (adjusting for age, sex, and race). Wilcoxon tests were used to compare differences in weekday versus weekend bedtime and wake time. Due to the small sample size, we did not correct for multiple comparisons. p Values < .05 were considered nominally significant.
Results
Participants included 9 adolescents with PAX6+/− (aged 10–19 years) as well as 25 healthy adolescents (aged 10–18 years) whose results we reported previously who served as a comparison group for this study (Hanish et al., 2017). Participant characteristics are shown in Table 1. Within the PAX6+/− group, there were no significant differences between adolescents with WAGR syndrome and those with isolated aniridia for total sleep questionnaire scores and sleep-onset latency (all ps > .15; data not shown). Due to these similarities and a research focus on PAX6+/− rather than on a specific condition, we combined participants with WAGR/11p deletion syndrome and isolated aniridia for all subsequent analyses.
Table 1.
Characteristics of Adolescents With PAX6+/− and Healthy Comparison Group.
| Characteristic | PAX6+/− (n = 9) | Healthy Comparison Group (n = 25)a | p Value |
|---|---|---|---|
| Age (years), mean ± SD (range) | 15.5 ± 3.3 (10–19) | 13.6 ± 2.3 (10–18) | .07 |
| Sex (% female) | 66.7 | 44.0 | .44 |
| Race/ethnicity (%) | 1.00 | ||
| Non-Hispanic Caucasian | 88.9 | 80.0 | |
| Other | 11.1 | 20.0 | |
| CASQ total score, mean ± SD | 34.6 ± 10.9 | 31.3 ± 8.7 | .39 |
| PROMIS sleep disturbance, mean ± SD | 45.5 ± 7.0 | 44.3 ± 7.2 | .67 |
| PROMIS sleep-related impairment, mean ± SD | 45.7 ± 10.2 | 50.2 ± 7.1 | .15 |
| Bedtime, median (25th to 75th percentile) | |||
| Overall | 22:19 (21:34–23:41) | 22:46 (22:25–23:48) | .11 |
| Weekday | 22:08 (21:23–23:33) | 22:18 (21:56–23:32) | .25 |
| Weekend | 22:40 (21:38–01:33) | 23:26 (23:04–00:14) | .14 |
| Wake time, median (25th to 75th percentile) | |||
| Overall | 7:09 (6:43–7:30) | 7:19 (7:00–8:00) | .11 |
| Weekday | 6:36 (6:19–7:10) | 7:05 (6:43–7:51) | .08 |
| Weekend | 7:24 (6:44–10:01) | 8:16 (7:51–8:43) | .25 |
| Overall sleep duration, median (25th to 75th percentile) | 7:42 (6:30–8:51) | 7:55 (7:19–8:15) | .86 |
| Sleep-onset latency (min), median (25th to 75th percentile) | 1.8 (1.2–3.9) | 2.4 (1.3–3.4) | .77 |
| Lights off to sleep (min), median (25th to 75th percentile) | 17.0 (6.1–74.4) | 7.9 (2.5–13.5) | .07 |
| Sleep efficiency (%), median (25th to 75th percentile) | 91.2 (89.8–92.2) | 91.1 (89.8–92.5) | .74 |
Note. Normally distributed variables are shown as mean ± SD and were analyzed using independent samples t test. Nonparametric data are shown as median (25th to 75th percentile) and were analyzed using Mann–Whitney U test. Percentages were compared using Fisher’s exact test. CASQ = Cleveland Adolescent Sleepiness Questionnaire; PROMIS = Patient Reported Outcomes Measurement Information System. aPreviously reported (Hanish, Lin-Dyken, & Han, 2017).
Subjective Sleep: Questionnaires
Total CASQ, PROMIS sleep-related impairment, and PROMIS sleep disturbance scores were not statistically different in adolescents with PAX6+/− versus the healthy comparison group (ps > .15; Table 1).
Objective Sleep: Actigraphy
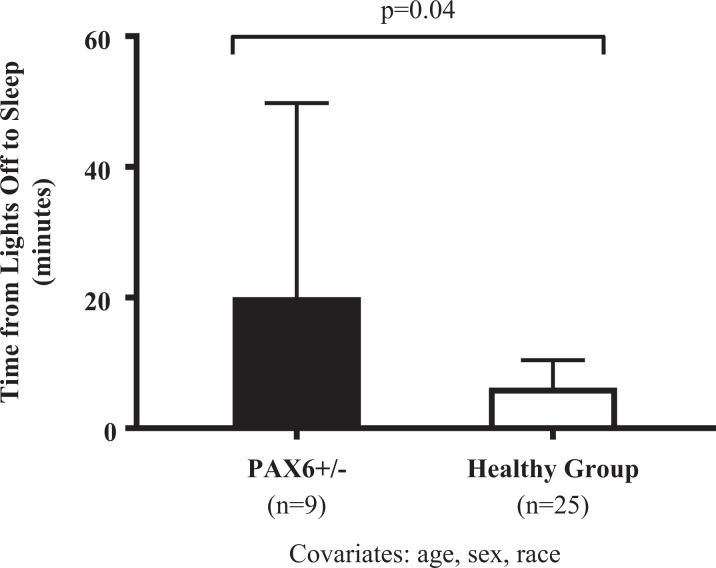
Overall bedtime (average of week), weekday bedtime, weekend bedtime, overall wake time (average of week), weekday wake time, weekend wake time, overall sleep duration, sleep-onset latency, and sleep efficiency were not statistically different in adolescents with PAX6+/− versus the healthy comparison group (ps > .08; Table 1). Both adolescents with PAX6+/− and the healthy comparison group on average had less than 8 hr per night (Table 1). Mean time from lights off to sleep onset was more than 2-fold higher in adolescents with PAX6+/− versus the healthy comparison group, but this difference did not reach statistical significance (p = .07; Table 1). After controlling for age, sex, and race, the adjusted mean time from lights off to sleep onset was nominally significantly higher in adolescents with PAX6+/− versus the healthy comparison group (adjusted mean [95% confidence interval]: 20.1 min [8.1, 49.8] vs. 6.2 min [3.7, 10.4], p = .04; Figure 1). We found no difference in weekday versus weekend bedtime or in weekday versus weekend wake time in adolescents with PAX6+/− (p = .26, p = .14, respectively).
Figure 1.
Greater adjusted time from lights off to sleep onset in adolescents with PAX6 haploinsufficiency (PAX6+/−) as compared to a healthy comparison group. Lights off to sleep onset was measured by actigraphy using Respironics Actiware 6 (Philips Respironics, Bend, OR). Covariates were age, sex, and race. Skewed data were normalized by log transformation, and back-transformed adjusted means with ±95% confidence intervals are shown. Nominal p value is shown.
Discussion
In the present study, we found that self-reported daytime sleepiness, sleep disturbance, and sleep-related impairment scores were similar in adolescents with PAX6+/− and the healthy comparison group. Likewise, using actigraphy, we also found that bedtime, wake time, overall sleep duration, sleep-onset latency, and sleep efficiency were similar in the two groups. However, time from lights off to sleep onset was higher in adolescents with PAX6+/− versus the healthy comparison group. We reported differences in weekday versus weekend bedtime and wake time in the healthy comparison group (Hanish et al., 2017), but we did not find such a difference in adolescents with PAX6+/−. The significant differences in weekday versus weekend bedtime and wake time we found in the comparison group are consistent with reports in the literature. Adolescent sleep patterns on weekends typically show a considerable delay and lengthening versus the weekdays, with sleep onset and offset occurring later (Wolfson & Carskadon, 1998).
We previously reported reduced pineal volume, melatonin secretion, and parent-reported sleep disturbance in children and adolescents with PAX6+/− (Hanish et al., 2016). Despite these abnormal findings, in the present study, we found no differences in self-reported daytime sleepiness, sleep disturbance, or sleep-related impairment in adolescents with PAX6+/− compared to the healthy comparison group. One possible explanation is confounding due to the overall inadequate sleep reported in general adolescent populations. According to the Academy of Sleep Medicine, adolescents physiologically require 8–12 hr of sleep per 24 hr (Paruthi et al., 2016). In recent decades, researchers, teachers, parents, and adolescents have consistently reported inadequate sleep throughout adolescence (J. Owens et al., 2014). Healthy People 2020 (2016) includes sleep health as an important topic area for public health, with one of the objectives specifically addressing the need to increase the proportion of adolescents who get a sufficient amount of sleep. In our sample, the majority of participants (67% of the adolescents with PAX6+/− and 56% of the healthy comparison group) slept less than 8 hr per night. Overall sleep deprivation in adolescents may thus have masked any differences between adolescents with PAX6+/− compared to the healthy comparison group. Another possible explanation is that the sample size for the PAX6+/− group was insufficient to identify differences in sleep measures.
Although we originally hypothesized that PAX6+/− would be associated with increased sleep-onset latency in comparison to healthy subjects, we found no significant difference between the groups. The actigraphy device and software we used in this study measured sleep-onset latency by calculating the time between rest and sleep intervals, which is based on the thresholds of activity count. However, although we found no differences using the automated algorithm to measure sleep-onset latency, we did find a significant difference using a custom interval that we created within the software program to assess time from lights out using the actigraph sensor with automated light thresholds to the onset of sleep. A light sensor provides useful information such as differentiating potential lights sources (e.g., overhead light or light from electronic media). In our custom calculation, we included lights off from all sources of light the sensor measures. This finding is also potentially clinically significant as the adjusted mean time from lights off to sleep was 6 min for the healthy comparison group, compared to 20 min in adolescents with PAX6+/−. Increased time from lights off to sleep may result in irregular time of sleep onset and reduced TST. For example, parents of children/adolescents with PAX6+/− have reported that bedtime generates a great deal of anxiety for their child, causing the child to leave his or her room to seek parent support. In addition, increased time from lights off to sleep may pose a safety risk in children/adolescents who need further adult supervision.
We also found that adolescents with PAX6+/− had more stable sleep patterns than those in the healthy comparison group, as demonstrated by the similar weekday versus weekend bedtime and wake time in the PAX6+/− group. One possible explanation for this greater sleep stability in adolescents with PAX6+/− may be greater parental involvement in bedtime routines due to parents’ perceptions of sleep difficulties. For example, one parent of an adolescent with PAX6+/− wrote, “I think she is a perfect candidate for your research, since she seems to have evidence of the sleep issues you’re studying.”
Although PAX6 haploinsufficiency is rare and minimal research has focused on the role of PAX6 in circadian regulation, investigators have studied irregular patterns of sleep–wake rhythm in children and adolescents with NDDs, another population with possible abnormalities in melatonin physiology. Authors have reported similar lower TST and longer sleep-onset latency in children (8–13 years of age) with Asperger’s syndrome (n = 16) compared with typically developing controls (n = 15; Allik, Larsson, & Smedje, 2008). While the extended sleep-onset latencies in NDD have been attributed to neurodevelopmental abnormalities, the etiology of the sleep alteration in patients with PAX6+/− may be attributable to the reduced melatonin concentration we previously reported in these patients (Hanish et al., 2016).
One limitation of our study is the small sample of adolescents with PAX6+/−. Both WAGR syndrome and isolated aniridia (prevalence 1/50,000–100,000) are rare disorders, however, and this study is the first to examine objective sleep measures in this population. There is no standard age range for defining adolescence. However, the World Health Organization defines adolescence as young people between the age of 10 and 19 years. We used a broad adolescent age range to aid in recruitment feasibility given the rare disease population. In future studies, researchers might consider using pubertal status within inclusion criteria. The small sample could have led to insufficient power to detect differences in sleep questionnaire scores and actigraphy variables. Due to the small sample and the complex interplay of both intrinsic and extrinsic factors including environmental factors (e.g., variability in school start times, extracurricular activities, and amount of parental supervision over bedtimes) in sleep disturbance (J. Owens et al., 2014), perhaps an expanded time frame (e.g., 2 weeks) would have generated more representative data. The inclusion criterion that adolescents must not have used sleep aids, including melatonin, in the 7 days preceding data collection also added to the recruitment challenges, as three potential participants were unable to stop melatonin for the purposes of the study. One parent described that her child had a “panic attack” when it came time to withhold melatonin and “sleep has been such an issue for her, we just can’t go back to how it was before.”
Although PAX6+/− is rare and recruitment in this population is difficult, common genetic polymorphisms that affect the function of PAX6 may be associated with insomnia in the general population (Ban, Kim, Seo, Kang, & Choi, 2011). Additional studies of these genetic variants and their impacts on self-reported sleep problems and sleep patterns could lead to genotype-specific treatment of insomnia such as using melatonin as a targeted therapy in PAX6-insufficient patients. In the present study, we used rare genetic disorders with biological vulnerability to sleep problems as a genotype–phenotype model. Knowledge of sleep-related phenotypes will assist in designing studies to manage sleep-related symptoms in adolescents.
Summary
This study is the first to explore sleep disturbance, sleep-related impairment, and sleep patterns in adolescents with PAX6+/− versus a healthy comparison group. We found significantly increased time from lights off to sleep in the adolescents with PAX6+/−, which may be a useful outcome measure for sleep interventions (e.g., melatonin replacement) in PAX6-insufficient patients and/or other populations with reduced melatonin concentrations. In this study, we used a population with a rare genetic condition as a model to expand our knowledge of sleep-related phenotypes with the goal of more broadly improving sleep function and managing or eliminating sleep-related symptoms in adolescents.
Acknowledgments
We thank the following individuals for their assistance: Jack Yanovski, Sheila Brady, Janet Williams, and Ann Berger.
Authors’ Note: The content does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. This study is registered at ClinicalTrials.gov (NCT00758108). J.C.H. is the recipient of an unrestricted research grant from Rhythm Pharmaceuticals. A.E.H. does not have any conflicts of interest to report.
Authors Contribution: Alyson E. Hanish contributed to conception, design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy. Joan C. Han contributed to conception, design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (1ZIAHD008898-02) and the National Institute of Nursing Research of the National Institutes of Health (NIH) and by the NIH Bench-to-Bedside Program.
References
- Abouzeid H., Youssef M. A., El Shakankiri N., Hauser P., Munier F. L., Schorderet D. F. (2009). PAX6 aniridia and interhemispheric brain anomalies. Molecular Vision, 15, 2074–2083. [PMC free article] [PubMed] [Google Scholar]
- Allik H., Larsson J. O., Smedje H. (2008). Sleep patterns in school-age children with Asperger syndrome or high-functioning autism: A follow-up study. Journal of Autism and Developmental Disorders, 38, 1625–1633. doi:10.1007/s10803-008-0543-0 [DOI] [PubMed] [Google Scholar]
- Baker E., Richdale A., Short M., Gradisar M. (2013). An investigation of sleep patterns in adolescents with high-functioning autism spectrum disorder compared with typically developing adolescents. Developmental Neurorehabilitation, 16, 155–165. doi:10.3109/17518423.2013.765518 [DOI] [PubMed] [Google Scholar]
- Ban H. J., Kim S. C., Seo J., Kang H. B., Choi J. K. (2011). Genetic and metabolic characterization of insomnia. PLoS One, 6, e18455 doi:10.1371/journal.pone.0018455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. M., Wielgus K. K., Young-McCaughan S., Fischer P., Farr L., Lee K. A. (2008). Methodological challenges when using actigraphy in research. Journal of Pain and Symptom Management, 36, 191–199. doi:10.1016/j.jpainsymman.2007.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S.,…PROMIS Cooperative Group. (2010). The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. Journal of Clinical Epidemiology, 63, 1179–1194. doi:10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Conduit R., Lockley S. W., Rajaratnam S. M., Cornish K. M. (2014). The relationship between sleep and behavior in autism spectrum disorder (ASD): A review. Journal of Neurodevelopmental Disorders, 6, 44 doi:10.1186/1866-1955-6-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estivill-Torrus G., Vitalis T., Fernandez-Llebrez P., Price D. J. (2001). The transcription factor Pax6 is required for development of the diencephalic dorsal midline secretory radial glia that form the subcommissural organ. Mechanisms of Development, 109, 215–224. [DOI] [PubMed] [Google Scholar]
- Fischbach B. V., Trout K. L., Lewis J., Luis C. A., Sika M. (2005). WAGR syndrome: A clinical review of 54 cases. Pediatrics, 116, 984–988. doi:10.1542/peds.2004-0467 [DOI] [PubMed] [Google Scholar]
- Goldman S. E., Richdale A. L., Clemons T., Malow B. A. (2012). Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders, 42, 531–538. doi:10.1007/s10803-011-1270-5 [DOI] [PubMed] [Google Scholar]
- Goldman S. E., Wang L., Fawkes D. B. (2014). Concordance of mother/child sleep patterns using actigraphy: Preliminary findings. Journal of Sleep Disorders—Treatment & Care, 3 doi:10.4172/2325-9639.1000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish A. E., Butman J. A., Thomas F., Yao J., Han J. C. (2016). Pineal hypoplasia, reduced melatonin and sleep disturbance in patients with PAX6 haploinsufficiency. Journal of Sleep Research, 25, 16–22. doi:10.1111/jsr.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanish A. E., Lin-Dyken D. C., Han J. C. (2017). PROMIS sleep disturbance and sleep-related impairment in adolescents: Examining psychometrics using self-report and actigraphy. Nursing Research, 66, 246–251. doi:10.1097/NNR.0000000000000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Measures. (2017). PROMIS: Dynamic tools to measure health outcomes from the patient perspective. Retrieved from http://www.healthmeasures.net/
- Healthy People 2020: Sleep Health. (2016). Washington, DC: U.S. Department of Health and Human Services, Office of Disease Prevention and Health Promotion; Retrieved from http://www.healthypeople.gov/2020/topics-objectives/topic/sleep-health [Google Scholar]
- Hirata I., Mohri I., Kato-Nishimura K., Tachibana M., Kuwada A., Kagitani-Shimono K.…Taniike M. (2015). Sleep problems are more frequent and associated with problematic behaviors in preschoolers with autism spectrum disorder. Research in Developmental Disabilities, 49–50, 86–99. doi:10.1016/j.ridd.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Jan J. E., Owens J. A., Weiss M. D., Johnson K. P., Wasdell M. B., Freeman R. D., Ipsiroglu O. S. (2008). Sleep hygiene for children with neurodevelopmental disabilities. Pediatrics, 122, 1343–1350. doi:10.1542/peds.2007-3308 [DOI] [PubMed] [Google Scholar]
- Lee H., Khan R., O’Keefe M. (2008). Aniridia: Current pathology and management. Acta Ophthalmologica, 86, 708–715. doi:10.1111/j.1755-3768.2008.01427.x [DOI] [PubMed] [Google Scholar]
- Lopez-Wagner M. C., Hoffman C. D., Sweeney D. P., Hodge D., Gilliam J. E. (2008). Sleep problems of parents of typically developing children and parents of children with autism. Journal of Genetic Psychology, 169, 245–259. doi:10.3200/GNTP.169.3.245-260 [DOI] [PubMed] [Google Scholar]
- Lunsford-Avery J. R., Krystal A. D., Kollins S. H. (2016). Sleep disturbances in adolescents with ADHD: A systematic review and framework for future research. Clinical Psychology Review, 50, 159–174. doi:10.1016/j.cpr.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malow B. A., McGrew S. G., Harvey M., Henderson L. M., Stone W. L. (2006). Impact of treating sleep apnea in a child with autism spectrum disorder. Pediatric Neurology, 34, 325–328. doi:10.1016/j.pediatrneurol.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Meltzer L. J. (2008). Brief report: Sleep in parents of children with autism spectrum disorders. Journal of Pediatric Psychology, 33, 380–386. doi:10.1093/jpepsy/jsn005 [DOI] [PubMed] [Google Scholar]
- Mitchell T. N., Free S. L., Williamson K. A., Stevens J. M., Churchill A. J., Hanson I. M.…Sisodiya S. M. (2003). Polymicrogyria and absence of pineal gland due to PAX6 mutation. Annals of Neurology, 53, 658–663. doi:10.1002/ana.10576 [DOI] [PubMed] [Google Scholar]
- National Institute of Nursing Research. (2016). The NINR strategic plan: Advancing science, improving lives. Retrieved from https://www.ninr.nih.gov/sites/www.ninr.nih.gov/files/NINR_StratPlan2016_reduced.pdf
- Novakova M., Paclt I., Ptacek R., Kuzelova H., Hajek I., Sumova A. (2011). Salivary melatonin rhythm as a marker of the circadian system in healthy children and those with attention-deficit/hyperactivity disorder. Chronobiology International, 28, 630–637. doi:10.3109/07420528.2011.596983 [DOI] [PubMed] [Google Scholar]
- Owens, J., Adolescent Sleep Working Group, & Committee on Adolescence. (2014). Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics, 134, e921–e932. doi:10.1542/peds.2014-1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. A., Spirito A., McGuinn M., Nobile C. (2000). Sleep habits and sleep disturbance in elementary school-aged children. Journal of Developmental and Behavioral Pediatrics: JDBP, 21, 27–36. [DOI] [PubMed] [Google Scholar]
- Paruthi S., Brooks L. J., D’Ambrosio C., Hall W. A., Kotagal S., Lloyd R. M.…Wise M. S. (2016). Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. Journal of Clinical Sleep Medicine, 12, 785–786. doi:10.5664/jcsm.5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rath M. F., Bailey M. J., Kim J. S., Ho A. K., Gaildrat P., Coon S. L.…Klein D. C. (2009). Developmental and diurnal dynamics of Pax4 expression in the mammalian pineal gland: Nocturnal down-regulation is mediated by adrenergic-cyclic adenosine 3’,5’-monophosphate signaling. Endocrinology, 150, 803–811. doi:10.1210/en.2008-0882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D. A., Frye R. E. (2014). Melatonin in autism spectrum disorders. Current Clinical Pharmacology, 9, 326–334. [DOI] [PubMed] [Google Scholar]
- Sivertsen B., Posserud M. B., Gillberg C., Lundervold A. J., Hysing M. (2012). Sleep problems in children with autism spectrum problems: A longitudinal population-based study. Autism: The International Journal of Research and Practice, 16, 139–150. doi:10.1177/1362361311404255 [DOI] [PubMed] [Google Scholar]
- Spilsbury J. C., Drotar D., Rosen C. L., Redline S. (2007). The Cleveland Adolescent Sleepiness Questionnaire: A new measure to assess excessive daytime sleepiness in adolescents. Journal of Clinical Sleep Medicine, 3, 603–612. [PMC free article] [PubMed] [Google Scholar]
- Stores G. (2016). Multifactorial influences, including comorbidities, contributing to sleep disturbance in children with a neurodevelopmental disorder. CNS Neuroscience & Therapeutics, 22, 875–879. doi:10.1111/cns.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. A., Schreck K. A., Mulick J. A. (2012). Sleep disruption as a correlate to cognitive and adaptive behavior problems in autism spectrum disorders. Research in Developmental Disabilities, 33, 1408–1417. doi:10.1016/j.ridd.2012.03.013 [DOI] [PubMed] [Google Scholar]
- van Kooten J. A., Terwee C. B., Kaspers G. J., van Litsenburg R. R. (2016). Content validity of the patient-reported outcomes measurement information system sleep disturbance and sleep related impairment item banks in adolescents. Health and Quality of Life Outcomes, 14, 92–016- 0496–5. doi:10.1186/s12955-016-0496-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C., Gruss P. (1991). Pax-6, a murine paired box gene, is expressed in the developing CNS. Development (Cambridge, England), 113, 1435–1449. [DOI] [PubMed] [Google Scholar]
- Wiggs L., Stores G. (1996). Severe sleep disturbance and daytime challenging behaviour in children with severe learning disabilities. Journal of Intellectual Disability Research: JIDR, 40, 518–528. [DOI] [PubMed] [Google Scholar]
- Wolfson A. R., Carskadon M. A. (1998). Sleep schedules and daytime functioning in adolescents. Child Development, 69, 875–887. [PubMed] [Google Scholar]