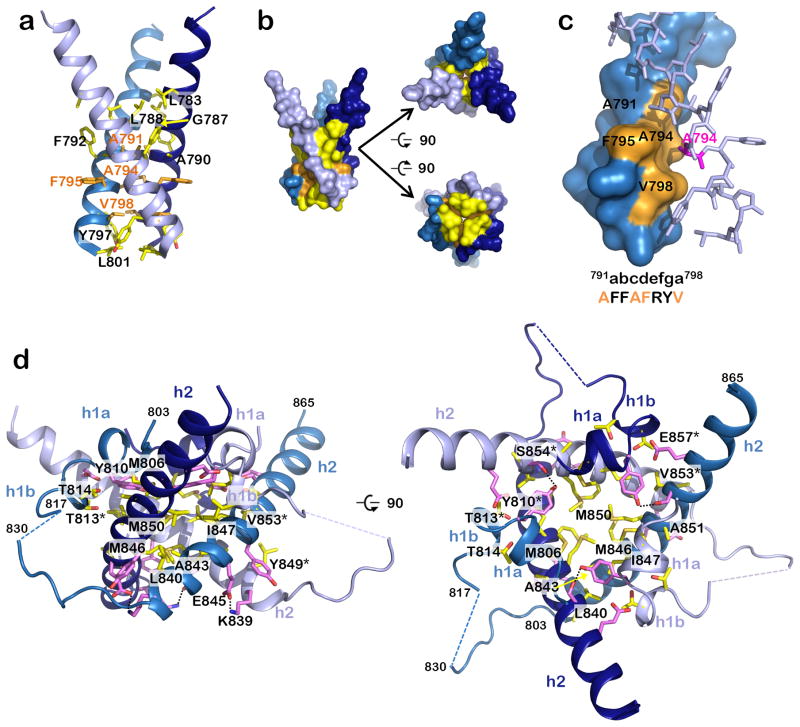
Figure 2.
Residues involved in formation of the trimeric MPR-TMD-CTD pedestal. (a) The TMD helices interact exclusively through hydrophobic residues (yellow and orange side chains), which mediate both hydrophobic contacts (side chain atoms) and van der Waals contacts (main chain atoms). (b) Surface representation of “a” emphasizing contact between the helices, in three views. (c) A subset of the TMD interface residues participates in a stabilizing knob-in-hole interaction, where A791, A794, F795, and V798 (orange) form a hole into which the A794 (pink) knob of the neighboring protomer fits. (d) Side and top views of the CTD showing residues that form salt bridges and hydrogen bonds (pink) or hydrophobic interactions (yellow). Salt bridges and hydrogen bonds are indicated by dotted black lines.