Abstract
The prevalence of diabetes, dyslipidemias, and high blood pressure is increasing worldwide especially in low and middle income countries. World Health Organization has emphasized the importance of the assessment of the magnitude of the specific disease in each country. We determined the prevalence and determinant factors of high blood pressure, hyperglycemia, dyslipidemias and metabolic syndrome in Ethiopia. A community based survey was conducted from -April to June 2015 using WHO NCD STEPS instrument version 3.1. 2008. Multistage stratified systemic random sampling was used to select representative samples from 9 regions of the country. A total of 10,260 people aged 15–69 years participated in the study. Blood pressure (BP) was measured for 9788 individuals. A total of 9141 people underwent metabolic screening. The prevalence of raised blood pressure (SBP ≥140 and/or DBP ≥ 90 mmHg) was 15.8% (16.3% in females and 15.5% in males). The prevalence of diabetes mellitus (FBS ≥ 126 mg /dl) including those on medication was 3.2% (3.5% males and 3.0% females). The prevalence of impaired fasting glucose was 9.1% with ADA criteria and 3.8% with WHO criteria. Hypercholesterolemia was found in 5.2%, hypertriglyceridemia in 21.0%, high LDL cholesterol occurred in 14.1% and low HDL cholesterol occurred in 68.7%. The prevalence of metabolic syndrome using IDF definition was 4.8% (8.6% in females and vs. 1.8% in males). Advanced age, urban residence, lack of physical exercise, raised waist circumference, raised waist hip ratio, overweight or obesity, and total blood cholesterol were significantly associated with raised blood pressure (BP) and diabetes mellitus. Increased waist- hip ratio was an independent predictor of raised blood pressure, hyperglycemia and raised total cholesterol. Our study showed significantly high prevalence of raised blood pressure, hyperglycemia and dyslipidemia in Ethiopia. Community based interventions are recommended to control these risk factors.
Introduction
According to the World Health Organization (WHO), 2017 report, Non- Communicable Diseases (NCDs) kill 40 million people. Three quarters of NCD deaths (28 million) occur in low and middleincome countries. Cardiovascular diseases account for most NCD deaths (17.7 million people) annually, followed by cancers (8.8 million), respiratory diseases (3.9 million), and diabetes (1.6 million) [1]. Globally blood pressure is the leading metabolic risk factor in terms of attributable deaths accounting for 18%, followed by overweight and obesity and raised blood glucose[2]. Chronic NCDs are rising fastest among lower–income countries [3–6]. The federal Ministry Health of Ethiopia established a National Strategic Action Plan for Non–Communicable Disease in Ethiopia (2014–2016), and developed national treatment guidelines and training materials on major NCDs like hypertension and diabetes. The national WHO STEPS survey was undertaken by the Federal Ministry of Health (FMOH) as part of a situational analysis of NCD risk factors to provide baseline data for subsequent interventions [7–10].
According to the Report of International Diabetes Federation (IDF) 2015 [11], the world- wide prevalence of diabetes mellitus among age group 20–79 was 8.8% (415 million people). WHO estimates that globally, high blood glucose is the third highest risk factor for premature mortality, after high blood pressure and tobacco use [12]. The regional prevalence of diabetes in the African population was estimated to be 2.1–6.7%. Ethiopia is estimated to have the fourth highest number of diabetes in the African region (1.3 million diabetes) [11].
The global prevalence of raised BP in adults aged 18 years and over was around 22% in 2014 (with highest prevalence being in Africa, at 30% for all adults combined [12](1. Hypertension has shown a rapid increase in prevalence affecting significant numbers of individuals in sub- Saharan Africa [13] (with a prevalence in the range of 25.4% to 41.1% in men and 27.2% to 38.7% in women [14] (. Different studies in Ethiopia showed a prevalence of hypertension of 19.6% (23.5% in urban population and 14.7% in rural/urban population), prevalence of in males and females was 20.6% and 19.2% respectively [15]. A study done in Tikur Anbessa specialized Hospital on stroke patients identified hypertension as a major risk factor in 69.3% of patients[16] (, Other studies also showed that hypertensive heart disease is the second most common diagnosis in the cardiac clinics[17–19]. A study from Nigeria, like Ethiopia, showed hypertension was more prevalent in the urban than rural dwellers with rates of 32.7% and 12.9% respectively[20].
Dyslipidemia (total cholesterol > 200 mg/dl, triglycerides > 150 mg /dl, LDL–C > 130 mg/l, HDL-C < 40 mg/dl; male, HDL–C < 50 mg/d; female) is one of the most important modifiable risk factors for the development of coronary heart disease[21]. According to a WHO estimate, globally the prevalence of raised total cholesterol is estimated to be 39%, and a third of ischemic heart disease is attributable to this risk factor[22]. The prevalence of dyslipidemias varies from country to country. In the USA,52% of adults had lipid abnormalities[23]. In Chinese subjects the prevalence of at least one type of abnormal lipid concentration was 64.4%[24]. In Nigeria, the prevalence of dyslipidemias ranged from 60% among apparently healthy Nigerians to 89% among diabetic Nigerians[25]. The prevalence of metabolic syndrome is estimated to be around 20–25 percent of the population and it doubled the risk to death and tripled the risk of cardiovascular disease compared to people without the syndrome[26,27]. Different studies have identified various sociodemographic factors like age, gender, place of residence and behavioral factors like excess alcohol intake, smoking and lack of physical activities as determinants of hypertension, diabetes and dyslipidemias[20,28,29].
So far there was no nationally representative study on NCD metabolic risk factors therefore this study as part of the National STEPS survey was aimed to determine the prevalence and determinant factors of high blood pressure, raised blood glucose and dyslipidemia and metabolic syndrome in Ethiopia.
Methods and materials
Study setting and period
The National WHO Steps survey was conducted in the 9 regions and two city administrations (Addis Ababa and Dire Dawa) of Ethiopia from mid-April to June 2015.
Study population
The national target population for this survey included all men and women age 15–69 years old who had lived at their current place of residence for at least six months. This target population included all people who considered Ethiopia to be their primary place of residence regardless of their citizenship status. Individuals who were not a permanent resident of Ethiopia, who were institutionalized-including people residing in hospitals, prisons, nursing homes, and other similar institutions or residents whose primary residences are military camps or dormitories, critically ill, mentally disabled and those with some type of physical disability that is not suitable for physical measurement were excluded from this study.
Sample size determination and sampling procedure
“A single population-proportion formula was used to determine the sample size. To adjust for the design effect, a complex sampling design effect coefficient of 1.5 was used to compute the sample size. To have an adequate level of precision for each age-sex estimate and place of residence, the sample was multiplied by the number of age-sex and place of residence groups for which the estimates were reported. Thus, Z-score = 1.96; proportion = 35.2%; this proportion is a comparative analysis on an estimated prevalence of raised blood pressure in Ethiopia according to countries by the WHO 2008, marginal error = 0.04 [30] design effect = 1.5; age-sex estimate and place of residence—sex estimate = 10 groups, and non-response rate = 20%. A total of 513 Enumeration Areas (EAs) were covered nationwide. Stratifying the sampling design, there were 404 rural EAs and 109 urban EAs. Taking into account the cost of the study and the level of precision—20 Households (HHs) per EA were selected using systematic random sampling and one eligible individual from each HH was selected using the Kish method (a random selection of eligible individuals at HH level). The final sample size was calculated to be 10,260 HHs (10,260 study participants)”. Reference 9 Annexed,WHO steps summary report,Ethiopia,2015 (see S1 Appendix). The survey was conducted using the WHO NCD STEPS instrument version 3.1. 2008[31] Step 1, Questionnaires based on interview on socio demographic data and behavioral status. Step 2, Physical measurement Step 3: Biochemical measurement of, fasting blood glucose, triglycerides, cholesterol, LDL and HDL (Laboratory procedures are annexed (see S2 Appendix). Additional optional questions were added to the instrument because they were locally relevant (see S3 Appendix)
Data collection and management
Data collection was done simultaneously at the 9 regions and 2 city administrations by trained nurses and lab professionals during a face to face interview, using s standardized questionnaire. Data were collected digitally using personal digital assistants (PDAs, eSTEPs software was used to design and program the data collection tools in the PDAs). The use of the software and PDAs to collect the data helped to generate the final dataset quickly following the completion of data collection. The collected datasets were stored in the device as well as the memory card in redline markup language (rml) format. The rml files from the PDAs were transferred to the supervisors’ tablet computers via the Windows Mobile Device Centre. The files were then transferred to a central server located at Ethiopian Public Health Institute (EPHI), Addis Ababa via Internet file streaming system (IFSS) software. IFSS is an application that connects to and exchanges data with the server component. Supervisors managed tablets supported by internet (EVIDEO) and run the IFSS icon (IfssClientPC.exe) located in their desktop to send all the updated data files to central server by entering their user name and password. Finally, IFSS automatically performed packaging, file delivery and receipt of incoming files. Data was managed using Excel, SPSS and Stata software.
Physical measurement and biochemical analysis
Physical measurements
Blood pressure and heart rate were measured for all survey participants whereas body weight, height, waist circumference, and hip circumference were measured for all survey participants except pregnant women. Body weight and height was measured with the electronic Growth Management Scale. It measures body weight and height, and calculates body mass index (BMI) (Body weight (kg)/ Body height/ (m2)). A BMI ≥ 25 indicates that a person is overweight, while a BMI ≥ 30 indicates that a person is obese. Waist circumference was measured by placing a tape measure around the bare abdomen at the midpoint between the lower margin of the last palpable rib and the top of iliac crest of the hip bone. Abdominal obesity was defined as a waist circumference of ≥94 cm for men and ≥80 cm women[32].
Blood pressure and heart rate measurements were taken three times on the right arm of the survey participants in a sitting position, using a Boso-Medicus Uno instrument with a universal cuff and automatic blood pressure and heart rate monitor. The mean of three measurements was taken for analysis. The measurements were taken after the participant had rested for 15 minutes, with three minutes of rest between the measurements (maximum deviation of cuff pressure measurement ± 3 mmHg, and of pulse rate display ± 5%).
Raised blood pressure was defined as: systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, or currently taking medication for hypertension. The percentage of respondents with treated and/or controlled raised blood pressure among those with raised blood pressure (SBP ≥140 and or DBP ≥ 90 mmHg) or currently taking medication for raised blood pressure was categorized as follows: For those taking medication as SBP <140 mmHg and DBP <90 mmHg (controlled hypertension) and SBP ≥140 mmHg and/or DBP ≥90 mmHg (uncontrolled hypertension) and for those not taking medication as SBP ≥140 mmHg and/or DBP ≥90 mmHg.
Biochemical analysis
Laboratory tests were performed for blood glucose, total cholesterol and HDL cholesterol using CardioCheck PA Analyser and for triglycerides using Cobas Integra 400 Plus (Roche Diagnostics GmbH, Mannheim, Germany) clinical chemistry analyzer. (Annexed) Concentrations of glucose (raised blood glucose level and percentage of respondents currently on medication for raised blood glucose), and lipid profile (total cholesterol, triglycerides, LDL and HDL cholesterol) were measured the next day after STEPS 1 and 2 of the data collection. Blood tests were performed for all survey respondents using a CardioChek PA Analyzer, after eight hours fasting.
WHO/IDF 2006[33] recommend that venous glucose should be the standard method for measuring and reporting glucose concentration in blood. However in recognition of wide use of capillary sampling, especially in under–resourced countries, conversion values for capillary plasma glucose are provided for post load values, fasting values for venous and capillary plasma glucose are identical[33].
Hyperglycemia is defined as plasma venous value: ≥ 7.0 mmol/L (126 mg/dl) and diabetes on medication, impaired fasting glucose (IFG—110–125 mg/dl (WHO criteria,) or 100–125 mg/dl, (ADA criteria), and normal blood glucose (< 110 mg /dl (WHO criteria) or < 100 mg/dl (ADA Criteria). dyslipidemia is defined as: (total cholesterol (≥200 mg/dl, triglycerides (≥ 150 mg/dl,LDL (≥130mg/dl and HDL cholesterol (40 mg/dl in male, < 50 mg/dl in female). In accordance with the IDF criteria, subjects were classified as having metabolic syndrome(MetS) if participants had abdominal obesity (defined as waist circumference of ≥94 cm for men and ≥80 cm women) plus two of any of the following risk factors: (1) raised TG level (≥150 mg/dL); (2) reduced HDLC (<40 mg/dL in males and <50 mg/dL in females); (3) raised blood pressure (systolic BP ≥130 or diastolic BP ≥85 mmHg) or treatment of previously diagnosed hypertension; (4) raised FG (≥100 mg/dL[32]
Data analyses
Descriptive weighted analysis was done along with complex sample analysis, and bivariate and multivariate analysis were conducted for raised blood pressure, raised blood glucose, and raised total cholesterol and metabolic syndrome. Further statistical analyses were done by using chi-squared tests and logistic regression models. Chi-squared tests were used for testing association of independent variables with the dependent variable. All factors with a p-value <0.05 in the bivariate analysis were further entered into the multivariate logistic regression model to control for confounding effects. Odds ratios (OR) with 95% confidence intervals (CI) were calculated. Statistical significance was accepted at the 5% level (p<0.05).
Ethical considerations
The study protocol was reviewed and approved by Institution Review Board of Ethiopian Public Health Institute (EPHI) and National Ethics Review Committee of Ministry of Science and Technology. Informed consent was obtained from each participant and consent obtained from parents and guardians for those participants between age 15–17. Adequate explanation was given to them on the survey procedure, risk, benefit, confidentiality, and the right to withdraw from the study. Individuals with high blood pressure, hyperglycemia and hypercholesterolemia were advised on prevention strategies by the survey team and also to visit the nearby health institution for further management.
Results
Prevalence of raised blood pressure and determinant factors
Overall BP was measured for 9788 individuals of which 8667(88.5%) were from rural areas and 5817(59.4%) were women. The overall prevalence of raised blood pressure (SBP ≥140 and/or DBP ≥ 90 mmHg) was 15.8% with slightly higher prevalence in females 16.3% versus (15.5%) in males. There was a progressive increase in prevalence of raised BP with increasing age of the participants. The urban prevalence of raised BP was 19.7% and rural prevalence was 14.9%. Among those with raised BP 42.7% had combined systolic and diastolic raised BP, 33.5% had isolated diastolic raised BP and 23.8% had isolated systolic raised BP. The prevalence of stage2 hypertension (> 160/90mmHg) was 4.4%. Of those with previous diagnosis of hypertension and on drug treatment, 53.4% had controlled BP (<140/90).
Prevalence of hyperglycemia
As shown in Table 1 a total of 9141 people underwent metabolic screening, 3842 were males and 5299 were females. The overall prevalence of diabetes mellitus (≥ 126 mg /dl) and previously diagnosed diabetes on medication was 3.2% (3.5% males and 3.0% females), with the majority between the age of 35 to 65 years of age. Few participants were aged 65–69 years. Prevalence of hyperglycemia increased with increasing age group of 15–24 to 64 yrs. (2.8% to 4.9%). About 0.5% of the study participants were reported as having diabetes on medication. Among the risk factors 6.7% were overweight and 8% were obese, raised waist circumference occurred in 5.6% of all participants.
Table 1. Prevalence of raised blood pressure, hyperglycemia and their determinants, WHO STEPs study, 2015, Ethiopia.
| Variables | Prevalence of raised blood pressure or taking medication | Prevalence of hyperglycaemia or currently taking medication | |||
|---|---|---|---|---|---|
| N | % | n | % | ||
| Sex | Male | 673 | 15.5% | 135 | 3.3% |
| Female | 1094 | 16.3% | 155 | 3.0% | |
| Total | 1767 | 15.8% | 290 | 3.2% | |
| Age Group | 15–24 | 210 | 9.1% | 38 | 2.6% |
| 25–34 | 425 | 15.0% | 65 | 2.6% | |
| 35–44 | 431 | 19.2% | 62 | 3.9% | |
| 45–54 | 319 | 22.2% | 55 | 4.3% | |
| 55–64 | 260 | 33.6% | 51 | 4.9% | |
| 65+ | 122 | 36.9% | 19 | 4.6% | |
| Total | 1767 | 15.8 | 290 | 3.2 | |
| Locality | Urban | 659 | 19.7% | 112 | 3.2% |
| Rural | 1108 | 14.9% | 178 | 3.2% | |
| Current alcohol use | Yes | 662 | 16.4% | 96 | 3.9% |
| No | 87 | 19.1% | 14 | 4.5% | |
| BMI | Underweight | 258 | 9.5% | 68 | 3.9% |
| Normal | 1125 | 17.0% | 157 | 2.6% | |
| Overweight | 257 | 36.3% | 48 | 6.7% | |
| Obese | 102 | 45.3% | 16 | 8.2% | |
| Quartiles of income | Q1 | 351 | 18.1% | 45 | 2.9% |
| Q2 | 286 | 14.6% | 44 | 2.2% | |
| Q3 | 268 | 15.0% | 41 | 2.6% | |
| Q4 | 333 | 17.6% | 55 | 3.3% | |
| Physical Activity Level | Low level | 393 | 18.2% | 76 | 4.8% |
| Moderate Level | 332 | 16.0% | 68 | 4.2% | |
| High Level | 1033 | 15.4% | 144 | 2.6% | |
| Raised waist circumference | Normal | 1442 | 15.4% | 180 | 2.9% |
| Raised | 303 | 43.3% | 109 | 5.6% | |
| Waist hip ratio level | Normal WHR | 987 | 13.9% | 142 | 4.3% |
| Raised WHR | 758 | 22.7% | 289 | 3.2% | |
| Raised Blood Pressure | Normal | NA | NA | 202 | 3.1% |
| Raised | NA | NA | 88 | 3.7% | |
| Raised blood glucose | No | 1678 | 15.8% | NA | NA |
| Yes | 89 | 18.9% | NA | NA | |
| Total Cholesterol level | <200 | 1494 | 16.0% | 200 | 1.9% |
| > = 200 | 176 | 20.6% | 88 | 30.2% | |
| Total | 1670 | 15.8% | 288 | 3.3% | |
Table 2, showed subgroup analysis of blood sugar values of WHO Steps 2015, Ethiopia compared to IDF /ADA criteria
Table 2. Fasting blood glucose level (WHO/IDF, 2006 vs ADA 2003) by background characteristics, Ethiopia Steps 2015.
| Variables | Blood glucose level Based on ADA/ IDF/WHO | Blood glucose level based on WHO | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (<100) | Impaired Fasting Glucose(100–125 mg/dl) | Hyperglycemia (> = 126 mg/dl) | Normal (<110) | Impaired Fasting Glucose(110–125 mg/dl) | |||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Sex | Male | 3075 | 87.7% | 373 | 8.8% | 127 | 3.5% | 3298 | 92.7% | 150 | 3.8% |
| Female | 4264 | 87.5% | 546 | 9.6% | 139 | 3.0% | 4574 | 93.1% | 236 | 3.9% | |
| Total | 7339 | 87.6% | 919 | 9.1% | 266 | 3.3% | 7872 | 92.9% | 386 | 3.8% | |
| Age Group | 15–24 | 1717 | 88.1% | 191 | 9.1% | 38 | 2.8% | 1832 | 93.8% | 76 | 3.4% |
| 25–34 | 2278 | 89.6% | 231 | 7.8% | 61 | 2.6% | 2412 | 93.9% | 97 | 3.5% | |
| 35–44 | 1596 | 85.6% | 214 | 10.2% | 61 | 4.1% | 1720 | 91.5% | 90 | 4.3% | |
| 45–54 | 972 | 86.6% | 144 | 9.2% | 48 | 4.2% | 1051 | 91.5% | 65 | 4.3% | |
| 55–64 | 530 | 83.9% | 97 | 11.6% | 42 | 4.5% | 588 | 90.6% | 39 | 4.9% | |
| 65+ | 246 | 83.6% | 42 | 12.1% | 16 | 4.3% | 269 | 88.9% | 19 | 6.8% | |
| Total | 7339 | 87.6% | 919 | 9.1% | 266 | 3.3% | 7872 | 92.9% | 386 | 3.8% | |
| Locality of the respondents | Urban | 1905 | 86.6% | 251 | 10.4% | 94 | 3.0% | 2042 | 92.7% | 114 | 4.3% |
| Rural | 5434 | 87.8% | 668 | 8.9% | 172 | 3.3% | 5830 | 93.0% | 272 | 3.7% | |
| Total | 7339 | 87.6% | 919 | 9.1% | 266 | 3.3% | 7872 | 92.9% | 386 | 3.8% | |
Dyslipidemia: Total Cholesterol ≥ 200 mg/dl, Triglyceride ≥ 150 mg /dl, LDL–C > 130 mg/l, HDL-C < 40 mg/dl–Male, HDL–C < 50 mg/d–Female.
According to IDF criteria, about 939 (9.1%) of the study participants were found to have impaired fasting glucose (IFG 100–125 mg/dl) compared to WHO criteria (3, 8%), i.e intermediate hyperglycemia. Among these 8.8% of them were males and 9.6% were females; 10.4% lived in urban area and 8.9% lived in rural Ethiopia. Prevalence of IFG increased from 9.1% in age range of 15–24, to 12.1% in age range of 65 and above.
Prevalence of raised total cholesterol
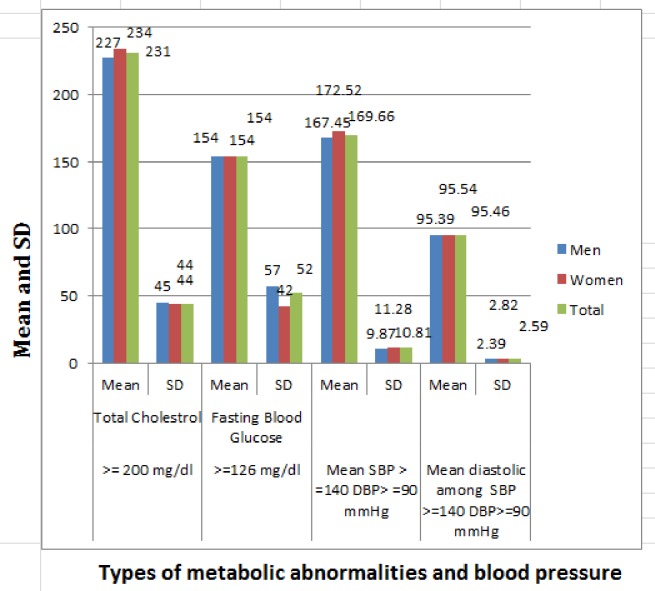
Hypercholesterolemia was found in 5.2%, of the study population which differs by sex, age group, area of residence, and other clinical and behavioral characteristics of study participants (Table 3, “Fig 1”). Low HDL–cholesterol and hypertriglyceridemia were the more prevalent dyslipidemias in the study participants. The mean levels of total cholesterol within participants having raised total cholesterol was 231±44mg/dl (“Fig 2”).
Table 3. Prevalence of raised total cholesterol level and metabolic syndrome, and their determinants WHO STEPS survey, Ethiopia 2015.
| Variables | Prevalence of raised total Cholesterol (> = 200 mg/dl) | Prevalence of metabolic syndrome * | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Sex | Male | 165 | 3.9% | 114 | 1.8% |
| Female | 421 | 6.8% | 643 | 8.6% | |
| Total | 586 | 5.2 | 757 | 4.8% | |
| Age Group | 15–24 | 84 | 4.1% | 59 | 1.6% |
| 25–34 | 131 | 4.3% | 144 | 3.6% | |
| 35–44 | 141 | 6.2% | 219 | 7.8% | |
| 45–54 | 122 | 8.3% | 171 | 9.8% | |
| 55–64 | 81 | 8.9% | 114 | 10.1% | |
| 65+ | 27 | 3.3% | 50 | 10.8% | |
| Total | 586 | 5.2 | 757 | 4.8% | |
| Locality | Urban | 230 | 7.1% | 437 | 11.7% |
| Rural | 356 | 4.8% | 320 | 3.2% | |
| Current alcohol use | Yes | 236 | 7.1% | 236 | 4.3% |
| No | 17 | 4.7% | 47 | 8.3% | |
| BMI | Underweight | 99 | 4.8% | 36 | 1.0% |
| Normal | 372 | 4.9% | 374 | 3.9% | |
| Overweight | 83 | 11.3% | 240 | 29.7% | |
| Obese | 29 | 8.4% | 107 | 41.7% | |
| Quartiles of income | Q1 | 76 | 4.2% | 119 | 4.5% |
| Q2 | 83 | 3.3% | 123 | 4.6% | |
| Q3 | 112 | 6.1% | 116 | 4.7% | |
| Q4 | 132 | 6.7% | 188 | 6.8% | |
| Physical Activity Level | Low level | 140 | 6.8% | 240 | 9.9% |
| Moderate Level | 121 | 6.7% | 159 | 6.0% | |
| High Level | 322 | 4.6% | 356 | 3.6% | |
| Raised waist circumference | Normal | 371 | 4.6% | 0 | 0.0% |
| Raised | 214 | 9.4% | 757 | 39.8% | |
| Waist hip ratio level | Normal WHR | 339 | 5.0% | 181 | 1.3% |
| Raised WHR | 246 | 5.8% | 576 | 14.1% | |
| Raised Blood Pressure | Normal | 412 | 4.9% | 278 | 2.1% |
| Raised | 174 | 7.1% | 479 | 18.8% | |
| Raised blood glucose (> = 126 mg/dl) | No | 498 | 3.8% | 663 | 4.3% |
| Yes | 88 | 46.5% | 94 | 18.0% | |
| Total Cholesterol level | <200 | NA | NA | 631 | 4.7% |
| > = 200 | NA | NA | 122 | 12.3% | |
| Total | NA | NA | 753 | 4.8% | |
*Metabolic syndrome (MetS) based on with the IDF criteria, subjects were classified as having MetS if participants had abdominal obesity (defined as waist circumference of ≥94 cm for men and ≥80 cm women) plus two of any of the following risk factors: (1) raised TG level (≥150 mg/dL); (2) reduced HDLC (<40 mg/dL in males and <50 mg/dL in females); (3) raised blood pressure (systolic BP ≥130 or diastolic BP ≥85 mmHg) or treatment of previously diagnosed hypertension; (4) raised FG (≥100 mg/dL
Fig 1. Prevalence of dyslipidemia, WHO STEPS survey, Ethiopia 2015.
Fig 2. Mean and SD values of total cholesterol, blood glucose, diastolic and systolic blood pressure, Ethiopia NCD steps, 2015.
Prevalence of metabolic syndrome
Table 3 depicts that the overall prevalence of metabolic syndrome in this study was 4.8% (757/ 8673); higher in females than in males (8.6% vs. 1.8%). Prevalence is higher in urban population than rural population (11.7% vs. 3.2%), increasing with increasing age group from 1.6% in age group 15–24 to 10.8% in age group 65 and above, prevalence was very high in obese patients compared to normal individuals (41% vs. 3.9%) and individuals with low level physical activities compared to high level physical activities (9.9% vs. 3.6%).
Predictors of metabolic abnormalities
Demographic and clinical CVD risk factors were investigated as potential predictors for the metabolic abnormalities using unadjusted odds ratio (OR) and95% confidence interval (CI). All significant predictors were included in the multiple logistic regression analyses controlling for possible confounders to compute for adjusted OR and their 95% CI as an approximation of the adjusted relative risk. How well the model fit the data was estimated using the Hosmer–Lemeshow test of goodness of fit. All reported P-values were two tailed, and the level of statistical significance was set at 0.05. The results of both bivariate and multivariate logistic regression analyses of risk factors for the overall metabolic abnormalities are presented in Table 4. After controlling for confounders, our findings show that socio demographic and economic characteristics such as age group, gender and economic and other residential and clinical factors such as area of residence, waist circumference, and BMI were found to be independent predictors of metabolic abnormalities.
Table 4. Bivariate and multivariate analyses of demographic and clinical risk factors for raised blood pressure, raised blood sugar, raised total cholesterol level, Ethiopia NCD Steps 2015.
| Variable characteristics | Raised blood pressure | Hyperglycemia | Total Cholesterol level > = 200 | ||||
|---|---|---|---|---|---|---|---|
| COR1 [95%CI] | AOR2 [95%CI] | COR1 [95%CI] | AOR2 [95%CI] | COR1 [95%CI] | AOR2 [95%CI] | ||
| Bivariate | Multivariate | Bivariate | Multivariate | Bivariate | Multivariate | ||
| Sex | Male | 1 | 1 | 1 | 1 | ||
| Female | 1.14(1.02,1.26) | 1.06(0.92,1.22) | 2.47(1.91,2.58) | 2.25(1.63,3.11) | 2.14(1.79,2.57) | 1.97(1.96,1.98) | |
| Age Group | 15–24 | 1 | 1 | 1 | 1 | 1 | 1 |
| 25–34 | 1.62(1.36,1.93) | 1.47(1.19,1.82) | 1.32(.88,1.97) | 1.24(.76,2.03) | 1.18(.89,1.56) | 1.04(.75,1.42) | |
| 35–44 | 2.54(2.13,3.03) | 2.04(1.64,2.52) | 1.75(1.16,2.63) | 1.48(.90,2.45) | 1.80(1.36,2.38) | 1.49(1.08,2.04) | |
| 45–54 | 3.26(2.69,3.94) | 2.66(2.11,3.35) | 2.51(1.65,3.82) | 1.79(1.07,3.02) | 2.56(1.92,3.41) | 2.03(1.45,2.83) | |
| 55–64 | 5.55(4.51,6.83) | 4.01(3.11,5.18) | 4.26(2.78,6.54) | 3.20(1.88,5.45) | 3.01(2.19,4.15) | 1.98(1.35,2.901) | |
| 65+ | 6.11(4.68,7.96) | 4.88(3.55,6.73) | 3.55(2.02,6.24) | 3.04(1.58,5.85) | 2.16(1.38,3.39) | 1.70(1.02,2.84) | |
| Locality | Rural | 1 | 1 | 1 | 1 | 1 | 1 |
| Urban | 1.76(1.58,1.96) | 1.27(1.10,1.48) | 1.68(1.32,2.14) | 1.26(.92,1.75) | 1.87(1.58,2.23) | 1.23(.98,1.54) | |
| BMI | Underweight | 1 | 1 | 1 | 1 | 1 | |
| Normal | 1.67(1.44–1.93) | 1.60(1.34,1.89) | .81(.60,1.09) | .64(.46,.91) | 1.36(1.09,1.71) | 1.20(.93–1.55) | |
| Overweight | 4.11(3.36–5.02) | 2.39(1.83,3.10) | 2.20(1.51–3.22) | 1.12(.67,1.86) | 2.78(2.05,3.77) | 1.21(.82–1.79) | |
| Obese | 6.79(5.04–9.15) | 3.07(2.11,4.48) | 2.51(1.43,4.41) | .93(.46,1.92) | 3.37(2.16,5.25) | 1.22(.71,2.09) | |
| Quintiles of income | Q1 | 1 | 1 | 1 | 1 | 1 | |
| Q2 | .82(.67,.99) | .80(.65,.99) | 1.19(.80,1.77) | 1.20(.78,1.85) | 1.43(1.09,1.87) | 1.48(1.11,1.97) | |
| Q3 | 1.047(.81,1.3) | 1.01(.76,1.34) | .86(.45,1.65) | .71(.35,1.45) | 2.17(1.57,3.01) | 2.37(1.68–3.34) | |
| Q4 | 1.24(1.01,1.53) | 1.14(.90,1.44) | 1.20(.73–1.97) | 1.08(.64,1.82) | 1.79(1.31,2.44) | 1.61(1.15,2.24) | |
| Q5 | .98(.79,1.21) | .77(.61,.99) | 1.62(1.07,2.44) | 1.50(.96,2.34) | 1.39(1.00,1.92) | 1.09(.77,1.56) | |
| Physical Activity Level | Low | 1.37(1.23,1.59) | .96(.78,1.17) | 1.91(1.44,2.53) | 1.06(.71,1.57) | 1.61(1.31,1.97) | .99(.74,1.32) |
| Moderate | 1.17(1.02,1.34) | .94(.80,1.118) | 1.73(1.29,2.32) | .74(.52,1.05) | 1.40(1.12,1.73) | .87(.68,1.11) | |
| High L | 1 | 1 | 1 | 1 | 1 | 1 | |
| Frequency of adding salt | Never Add | 1 | 1 | 1 | 1 | 1 | 1 |
| Sometimes | 1.12(.98,1.28) | 1.04(.89,1.22) | 1.73(1.20,2.50) | 1.68(1.11,2.54) | 1.40(1.00,1.72) | 1.19(.92,1.55) | |
| Usually | .99(.83,1.17) | .87(.71,1.07) | 1.99(1.32,3.02) | 1.80(1.12,2.88) | 1.91(1.45–2.51) | 1.84(1.37,2.47) | |
| Raised waist circumference | Normal | 1 | 1 | 1 | 1 | 1 | 1 |
| Raised | 2.69(2.38,3.02) | 1.56(1.30,1.88) | 2.49(1.95,3.18) | 1.16(.79,1.71) | 2.51(2.10–2.99) | 1.647(1.27,2.14) | |
| Waist hip ratio level | Normal | 1 | 1 | 1 | 1 | 1 | 1 |
| Raised | 1.87(1.68,2.08) | 1.25(1.09,1.44) | 2.13(1.68,2.69) | 1.55(1.14,2.12)) | 1.61(1.36,1.91) | 1.02(.82,1.28) | |
| Raised Blood pressure | Normal | NA | NA | 1 | 1 | 1 | 1 |
| Raised | NA | NA | 1.96(1.52,2.54) | 1.26(.92,1.73) | 1.89(1.57,2.27) | 1.32(1.06,1.65) | |
| Raised Blood glucose (WHO) | <126 | 1 | 1 | NA | NA | 1 | 1 |
| > = 126 | 2.03(1.57,2.61) | 1.25(.91,1.72) | NA | NA | 6.98(5.35,9.11) | 4.2(3.02,5.84) | |
| Raised total cholesterol |
Normal | 1 | 1 | 1 | 1 | NA | NA |
| Raised | 1.68(1.40,2.01) | 1.35(1.08,1.68) | 6.98(5.35–9.11) | 4.36(3.15,6.05) | NA | NA |
|
1 Crude odds Ratio (COR).
2 Adjusted Odds ratio (AOR).
Among the non-modifiable risk factors, despite the preponderance of the female population in our samples, the prevalence of metabolic abnormality was not statistically different between the sexes; however, individuals aged ≥65 years were at increased risk of developing the metabolic abnormality (Table 4). On analysis of various socio-demographic and behavioral factors: age, urban residence, tobacco use, lack of physical exercise, alcohol intake, added salt, BMI were associated with raised BP. On multivariate logistic regression analyses urban residence, age, lack of physical exercise and BMI were strongly associated with raised BP (Table 4). Similarly, advanced age, urban residence, adding salt to food, low physical activities, raised waist circumference, raised waist hip ratio, overweight or obese, and having raised blood glucose were significantly associated with diabetes mellitus, and hypercholesterolemia. Our results also show that increased waist/hip ratio was independently predictive of the raised blood pressure, raised blood sugar and raised total cholesterol with (adjusted OR 1.25, 1.55 and 1.02) respectively. Similarly increased waist circumference was independently predictive of the raised blood pressure and raised total cholesterol with (adjusted OR 1.56, 1.64)) respectively.
Discussions
Our study is the first national community based survey on NCDs and one of the few WHO STEPS Surveys in Africa, which applied all the three instruments of STEPS tools. Our main findings showed prevalence of high blood pressure in 15.8%, with higher prevalence in urban population versus rural population (19.7% vs 14.9%), Vantu NCD steps survey in 2012 showed higher prevalence compared to our report (28.6%)[34]. Diabetes occurred in 3.2%, which is slightly lower than the IDF Country estimate for Ethiopia 2015 (3.4%)[11] which was derived by extrapolation from neighboring countries. Impaired fasting glucose occurred in 9.1% (ADA criteria) or in 3.8% (WHO criteria). Hypertriglyceridemia and low HDL cholesterol were more prevalent dyslipidemias, and metabolic syndrome occurred in 4.8% of study participants. Our study showed a lower prevalence of metabolic syndrome compared to reports from India (35.8%- 45.3%) and China (30.5%-31.5%)[35,36]. The prevalence of all metabolic cardiovascular risk factors was higher in urban than rural populations and progressively increased with advancing age. Finding obtained from community based studies from Indian population also showed CVS risk factors significantly increased with advancing age[37]. Increased waist hip ratio was independently predictive of the raised blood pressure, raised blood sugar and raised total cholesterol with (adjusted OR 1.25, 1.55 and 1.02) respectively. Similarly increased waist circumference was independently predictive of the raised blood pressure and raised total cholesterol with (adjusted OR 1.56, 1.64)) respectively. Previous report from Northern Ethiopia also showed gender, age and area of residency and education as risk factors for CVD[38]
Our study revealed considerably high prevalence of raised BP (15.8%), and is much lower than that of the 2014 WHO national estimate for raised BP in adults >18 years (28.8%) and the pooled national prevalence estimate (19.6%), but higher than that of Butajira (12% in men and 8% in women)[12,15,39].This difference is possibly attributed to the difference in place of residence and age composition of the study population. In this survey the prevalence of raised BP has been shown to increase with age with the highest prevalence being in those above 65 years of age. Elevation in both systolic and diastolic BP is the commonest form followed by isolated diastolic BP elevation. Other studies also showed more isolated diastolic BP than isolated raised systolic BP in younger individuals below 40 years of age[40,41] and the higher proportion of younger population in the study might have resulted in higher proportion of diastolic raised BP in our study. The Framingham Heart Study showed that DBP was a better predictor of future coronary heart disease events than SBP in adults <50 years of age; the reverse was true after 50 years of age underlying the importance of isolated diastolic hypertension[42]. There is an observed difference between rural and urban respondents. This has been demonstrated in several other studies related to more sedentary life style in urban dwellers[28,43] The urban prevalence of raised BP in our study is considerably lower than that of urban Addis Ababa (31.2% males; 28.5% female) and Bahr Dar25.1%[42,44]. A recent Study also showed higher prevalence (25%) among 2716 adults in Addis Ababa[45]. These differences can be explained by the differences in the level of urbanization with corresponding difference in the determinants of raised BP.
The prevalence of raised BP in our study is higher in females but on multivariate analysis there was no statistically significant difference in the prevalence of raised BP between males and females. Prevalence of raised BP was found to be higher in males than females in several studies until menopause [46]. The gender disparity in raised BP is believed to be due to difference in biological and behavioral factors including hormonal difference, obesity, cigarette smoking, alcohol consumption and physical activities. The apparently high prevalence of raised BP in females in our study could be due to significant difference in level of physical activity; men (318.2 minutes, 95% CI: 302.7–333.7) and women (236.2 minutes, 95% CI:223.1–249.3) and higher prevalence of obesity in females (2.0 versus 0.5%). Treatment of hypertension was found to be lower than that reported from other low income countries (2.8% versus 31.7%)(36),[47]. The survey identified several determinants of high BP; the strongest being age. Other determinants included place of residence, alcohol intake, cigarette smoking, khat use, BMI, raised BMI and FBG. Similar determinants were also reported in other studies[20,29,39] In the current community survey, the prevalence of diabetes mellitus was 3.2% (3.3% in males and 3.0% in females).
This was an epidemiological survey and repeat testing was not done, The prevalence of diabetes and pre diabetes was lower compared to reports from urban slums of Bangalore (12.33%, 11.57%) respectively[48]. They studied urban population age 35 and above whereas we included both rural and urban population age 15 -69years. The inclusion of younger people in our survey may have reduced the prevalence. The prevalence was more among the females than males similar to the later report. A population based prevalence survey in Austria showed a higher overall diabetes prevalence of 7.4%. Prevalence in men was 8.0% and in women was 6.8%. In addition, 17.4% of men and 15.45 of women had IGT or IFG[49]. Malawi STEPs survey 2009 [50]. showed a prevalence raised blood pressure in 32.9%, diabetes in 5.6%, and raised cholesterol in 8.7% compared to ours (15.8%, 3.2%, 5.2%) respectively.
Impaired fasting glucose is a prediabetes state. In the current study the prevalence of impaired fasting glucose (IFG) was 9.1% (ADA criteria) vs. 3.8% (WHO Criteria). Use of the ADA criteria more than doubled the prevalence of IFG. There are a number of differences in several diagnostic criteria of glucose intolerance between WHO and ADA. Increasing fasting plasma glucose in a non-diabetic range is associated with fatal and non-fatal cardiovascular disease[51,52]. WHO recommended the fasting plasma glucose cut point for IFG should remain at 6.1 mmol/L(110 mg/dl) (WHO,2006, Recommendation 4 and 5) and ADA proposed a lower blood sugar values (100 mg/dl) [33]. Incidence rates of diabetes are important consideration. Mauritius and Pima Indian data indicated that risk of progression of IFG in the additional people identified by ADA criteria is half that of WHO criteria (15% versus 30%) over 5 years [53,54]. Our study also showed a significant difference in the prevalence of IFG (9.1% vs 5.4%) compared to WHO vs ADA criteria[33] and identified risk groups for diabetes and cardiovascular diseases. In our study the prediabetes state was more common in females, urban dwellers, prevalence increased with advancing age, higher in overweight and obese individuals, and individuals with low physical activities. Such findings are warrants an early intervention to halt the progression to diabetes and cardiovascular disease.
Dyslipidemias are the major cardiovascular risk factors. Our study showed that among dyslipidemias, hypertriglyceridemia and low HDL-C occurred in 21% and 68% of participants respectively. The most prevalent dyslipidemia was low HDL- cholesterol, which suggests that participants may be prone to cardiovascular disease. A study from São Paulo Brazil also showed high prevalence of low HDL = C (59.74%)[55]. The ICMR-INDIAB study[56] reported 13.9% had hypercholesterolemia, 29.5% had hypertriglyceridemia 72.3% had low HDL and 11.8% had high LDL. Seventy-nine (79%) had abnormalities in one of the lipid parameters. Similar trends were seen in our study.
As already mentioned above, we have applied IDF definition and found a prevalence of metabolic syndrome of 4.8% of study participants. The prevalence of metabolic syndrome is variable depending on the definitions of metabolic syndrome. The prevalence of metabolic syndrome in African populations ranges from as low as 0% to as high as about 50% or even higher in certain population setting[57], In study conducted in Malaysia the prevalence of metabolic syndrome according to IDF, NCEP ATP III and WHO definitions were 22.9%, 16.5% and 6. 4% respectively[58]. Several reports showed a high prevalence of metabolic syndrome compared to our report (China 35.8% to 45.3%, India 35.8% to 45.3%)[35]. In our study metabolic syndrome was frequently occurred with its determinants like raised waist circumference, high blood pressure, raised blood glucose, hypercholesterolemia, and a low level of physical activity.
In general overweight and obesity are associated with an increased risk of developing hypertension and diabetes[59–61]. In multivariate analysis, our results also showed that increased waist hip ratio was independently predictive of the raised blood pressure, hyperglycemia and raised total cholesterol (adjusted OR 1.25, 1.55 and 1.02) respectively. Similarly, increased waist circumference was independently predictive of the raised blood pressure and raised total cholesterol with (adjusted OR 1.56, 1.64)). Such a finding is alarming in resource-limited settings like Ethiopia where screening programs for CVD risk factors are not a priority due to the high burden of infectious diseases.
Strength and limitations
Our study is the first nationwide large community based survey on metabolic risk factors of cardiovascular disease. In addition, the study findings can be generalized to the population because of the large sample size, sampling procedures, and high response rate of study participants. Some of the limitations were since it was a community survey, classification of diabetes and repeat blood glucose determination was not done as per recommendation of ADA.
Conclusions and Recommendations: The current community based WHO steps survey identified the prevalence of high blood pressure, diabetes, in 15.8% and 3.2% respectively. Hypertriglyceridemia and a low HDL were the most common dyslipidemias. Prevalence of IFG was 9.1% using ADA criteria more than double those identified by WHO criteria. Metabolic syndrome occurred in 4.8% of study population. Raised blood pressure, diabetes, dyslipIdemias, metabolic syndrome were more common in females, urban population and with advancing age. Increased waist hip ratio was an independent predictor of the raised blood pressure, hyperglycemia and raised total cholesterol; In general we recommend establishing a community based screening and intervention strategies on NCD’s modifiable risk factors and establishing efficient and sustainable health education system on life style modification.
Supporting information
(PDF)
(DOC)
(PDF)
Acknowledgments
The Ethiopia non-communicable diseases (NCDs) Step survey demonstrates the combined efforts of the Federal Ministry of Health (FMOH), development partners, professional associations, and Expert individuals without which these findings could not have been possible. The authors also would like to thank all the field staff involved in data collection and supervision for their crucial roles played in achieving the survey goal. The overall coordination and guidance provided by Health System Research Directorate of EPHI is also highly appreciated. Special thanks also go to the TB and HIV research directorate team who performed laboratory analyses for biological samples to make the report a reality and special thanks to Dr. Julia Lowe, Endocrinologist from University of Toronto, Canada for reviewing the draft and final manuscript. The institute would like to extend its sincere gratitude to the survey participants who cooperated and participated in the realization of the survey.
Data Availability
Summary data are available in S1 File. The National WHO Steps survey data underlying this study are National Data available from the Ethiopian Public Health Institute, Ethiopia. Interested, qualified researchers may submit queries related to data access to: Dr. Getachew Addis; Address: EPHI Scientific and EthicsReview Office, P.O Box 1242, Addis Ababa, Ethiopia; Office Phone: +251-118-685503/15; E-mail: get_ast@yahoo.com.
Funding Statement
Both World Health Organization (WHO) and United Nation International Children's Emergency Fund (UNICEF) supported the The WHO STEPS, Ethiopia, in terms of financial support, in kind and as Expert support. We have applied the Standard format of WHO STEPS Survey with minor modification. But the funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The overall organizer of the funding is the Federal Ministry Health Of Ethiopia. Mr. Abebe Belayneh, the head of the Health System and Reproductive Health Research Directorate is responsible for grant related activities.
References
- 1.World Health Organization (2017) Non-Communicable Diseases, Fact sheet.
- 2.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, et al. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH (2014) Global Status Report on Non-Communicable Diseases, World Health, 176.
- 4.Alwan A, Maclean DR, Riley LM, d'Espaignet ET, Mathers CD, et al. (2010) Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. Lancet 376: 1861–1868. doi: 10.1016/S0140-6736(10)61853-3 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (2013) Global action plan for the prevention and control of noncommunicable diseases 2013–2020.: Available from: http://apps.who.int/iris/bitstream/10665/94384/1/9789241506236_eng.pdf.
- 6.World Health Organization (2016) Global report on diabetes Swizerland, Geneva:WHO Press,. [Google Scholar]
- 7.Ethiopian Federal Ministry of Health (2014) National Strategic Action Plan for Prevention and Control of Non Communicable Diseases in Ethiopia 2014–2016.
- 8.Ethiopian Federal Ministry of Health (2016) National Comprehensive Guidelines for Clinical and Programmatic Management of Major Non-Communicable Diseases. 160–165.
- 9.World Health Organization (2016) Ethiopia Steps Report on Risk Factors for Non-Communicable Diseaes and Prevalence of Selected NCDs Ethiopia Steps Report On Risk Factors For Chronic Non-Communicable Diseases.
- 10.Ethiopian Federal Ministry of Health (2016) National Training guidelines on diabetes mellitus, hypertension Federal Democratic Republic of Ethiopia,: Ministry of Health. [Google Scholar]
- 11.International Diabetes Federation (IDF) (2015) DIABETES ATLAS Seventh Edition 2015, International Diabetes Federation 144 p. p.
- 12.World Health Organization (2009) Global Health Risks: Mortality and burden of disease attributable to selected major risks Bull World Health Organ; [Internet],. Available from: http://www.who.int/healthinfo/global_burden_disease/GlobalHealthRisks_report_full.pdf. [Google Scholar]
- 13.World Health Organization (2014) Global status report on noncommunicable diseases: “Attaining the nine global noncommunicable diseases targets; a shared responsibility”; Italy, editor. Geneva, Switzerland: Second Press WHO. [Google Scholar]
- 14.Kuller LH (2007) Epidemic hypertension in Sub-Saharan Africa. Hypertension 50: 1004–1005. doi: 10.1161/HYPERTENSIONAHA.107.095620 [DOI] [PubMed] [Google Scholar]
- 15.Addo J, Smeeth L, Leon DA (2007) Hypertension in sub-saharan Africa: a systematic review. Hypertension 50: 1012–1018. doi: 10.1161/HYPERTENSIONAHA.107.093336 [DOI] [PubMed] [Google Scholar]
- 16.Kibret KT, Mesfin YM (2015) Prevalence of hypertension in Ethiopia: a systematic meta-analysis. Public Health Reviews 36: 14 doi: 10.1186/s40985-015-0014-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alemayehu CM, Birhanesilasie SK (2013) Assessment of stoke patients: occurrence of unusually high number of haemorrhagic stroke cases in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Clin Med Res 2: 94–100. [Google Scholar]
- 18.Abdissa SG, Oli K, Feleke Y, Goshu DY, Begna DM, et al. (2014) Spectrum of cardiovascular diseases among Ethiopian patients at Tikur Anbessa Specialized University Teaching Hospital, Addis Ababa. Ethiop Med J 52: 9–17. [PubMed] [Google Scholar]
- 19.Belayneh AB, Teklie H, Tadesse M, Defar A, Getachew T, et al. (2015) Pattern and trend of medical admissions of patients of chronic non-communicable diseases in selected Hospitals in Addis Ababa, Ethiopia. American Scientific Research Journal for Engineering, Technology, and Sciences (ASRJETS) 13: 34–48. [Google Scholar]
- 20.Adediran Olufemi Sola OIC, Stephen Adeniyi Olasupo,Kayode Jimoh Ahmed, (2013) Hypertension prevalence in an Urban and Rural area of Nigeria. Journal of Medicine and Medical Sciences 4: 149–154. [Google Scholar]
- 21.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, et al. (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The lancet 364: 937–952. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization (2014) Overweight, Global health observatory:Deaths from NCDs.
- 23.Tóth PP, Potter D, Ming EE (2012) Prevalence of lipid abnormalities in the united states: the national health and nutrition examination survey 2003–2006. Journal of clinical lipidology 6: 325–330. doi: 10.1016/j.jacl.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 24.Sun G-Z, Guo L, Wang X-Z, Song H-J, Li Z, et al. (2015) Prevalence of atrial fibrillation and its risk factors in rural China: a cross-sectional study. International journal of cardiology 182: 13–17. doi: 10.1016/j.ijcard.2014.12.063 [DOI] [PubMed] [Google Scholar]
- 25.Oguejiofor O, Onwukwe C, Odenigbo C (2012) Dyslipidemia in Nigeria: prevalence and pattern. Annals of African medicine 11: 197 doi: 10.4103/1596-3519.102846 [DOI] [PubMed] [Google Scholar]
- 26.Dunstan DW, Zimmet PZ, Welborn TA, De Courten MP, Cameron AJ, et al. (2002) The rising prevalence of diabetes and impaired glucose tolerance: the Australian Diabetes, Obesity and Lifestyle Study. Diabetes care 25: 829–834. [DOI] [PubMed] [Google Scholar]
- 27.Isomaa B, Almgren P, Tuomi T, Forsén B, Lahti K, et al. (2001) Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes care 24: 683–689. [DOI] [PubMed] [Google Scholar]
- 28.Tesfaye F (2008) Epidemiology of cardiovascular disease risk factors in Ethiopia: the rural-ruban gradient: Epidemiologi och folkhälsovetenskap.
- 29.Kishore J, Gupta N, Kohli C, Kumar N (2016) Prevalence of hypertension and determination of its risk factors in rural Delhi. International journal of hypertension. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogah OS, Rayner BL (2013) Recent advances in hypertension in sub-Saharan Africa. Heart 99: 1390–1397. doi: 10.1136/heartjnl-2012-303227 [DOI] [PubMed] [Google Scholar]
- 31.Organization WH (2009) WHO STEPS Instrument for Chronic Disease. 1–12 p.
- 32.Alberti G, Zimmet P, Shaw J, Grundy S International Diabetes Federation. The IDF consensus worldwide definition of the Metabolic Syndrome International Diabetes Fundation publication; 2006: 2–24. [Google Scholar]
- 33.Organization WH (2006) Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF consultation World Hearth Org. [Google Scholar]
- 34.Roberts G, Tarivonda L, Bell C, Raj S, Latu R (2013) Vanuatu: NCD risk factors STEPs report.
- 35.Ravikiran M, Bhansali A, Ravikumar P, Bhansali S, Dutta P, et al. (2010) Prevalence and risk factors of metabolic syndrome among Asian Indians: a community survey. Diabetes research and clinical practice 89: 181–188. doi: 10.1016/j.diabres.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 36.Zuo H, Shi Z, Hu X, Wu M, Guo Z, et al. (2009) Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism-Clinical and Experimental 58: 1102–1108. doi: 10.1016/j.metabol.2009.04.008 [DOI] [PubMed] [Google Scholar]
- 37.Walia R, Bhansali A, Ravikiran M, Ravikumar P, Bhadada SK, et al. (2014) High prevalence of cardiovascular risk factors in Asian Indians: A community survey-Chandigarh Urban Diabetes Study (CUDS). The Indian journal of medical research 139: 252 [PMC free article] [PubMed] [Google Scholar]
- 38.Mengesha AB (2015) Epidemiology of Preventable Risk Factors for Non-communicable Diseases among Adult Population in Tigray, Northern Ethiopia. [Dissertation]: Addis Ababa Univeristy.
- 39.Tesfaye F, Nawi N, Van Minh H, Byass P, Berhane Y, et al. (2007) Association between body mass index and blood pressure across three populations in Africa and Asia. Journal of human hypertension 21: 28 doi: 10.1038/sj.jhh.1002104 [DOI] [PubMed] [Google Scholar]
- 40.Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, et al. (2005) Predictors of new-onset diastolic and systolic hypertension: the Framingham Heart Study. Circulation 111: 1121–1127. doi: 10.1161/01.CIR.0000157159.39889.EC [DOI] [PubMed] [Google Scholar]
- 41.Franklin SS, Jacobs MJ, Wong ND, Gilbert J, Lapuerta P (2001) Predominance of isolated systolic hypertension among middle-aged and elderly US hypertensives: analysis based on National Health and Nutrition Examination Survey (NHANES) III. Hypertension 37: 869–874. [DOI] [PubMed] [Google Scholar]
- 42.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, et al. (2001) Does the relation of blood pressure to coronary heart disease risk change with aging?: The Framingham Heart Study. Circulation 103: 1245–1249. [DOI] [PubMed] [Google Scholar]
- 43.Muluneh AT, Haileamlak A, Tessema F, Alemseged F, Woldemichael K, et al. (2012) Population based survey of chronic non-communicable diseases at gilgel gibe field research center, southwest Ethiopia. Ethiopian journal of health sciences 22: 7–18. [PMC free article] [PubMed] [Google Scholar]
- 44.Anteneh ZA, Yalew WA, Abitew DB (2015) Prevalence and correlation of hypertension among adult population in Bahir Dar city, northwest Ethiopia: a community based cross-sectional study. International journal of general medicine 8: 175 doi: 10.2147/IJGM.S81513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdissa SG, Feleke Y, Awol M (2015) Prevalence of hypertension and pre-hypertension in Addis Ababa, Ethiopia: A survey done in recognition of World Hypertension Day, 2014. The Ethiopian Journal of Health Development (EJHD) 29. [Google Scholar]
- 46.Everett B, Zajacova A (2015) Gender differences in hypertension and hypertension awareness among young adults. Biodemography and social biology 61: 1–17. doi: 10.1080/19485565.2014.929488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, et al. (2016) Global Disparities of Hypertension Prevalence and ControlClinical Perspective: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 134: 441–450. doi: 10.1161/CIRCULATIONAHA.115.018912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dasappa H, Fathima FN, Prabhakar R, Sarin S (2015) Prevalence of diabetes and pre-diabetes and assessments of their risk factors in urban slums of Bangalore. Journal of family medicine and primary care 4: 399 doi: 10.4103/2249-4863.161336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunstan DW, Zimmet PZ, Welborn TA, Cameron AJ, Shaw J, et al. (2002) The Australian diabetes, obesity and lifestyle study (AusDiab)—methods and response rates. Diabetes research and clinical practice 57: 119–129. [DOI] [PubMed] [Google Scholar]
- 50.Ministry of Health Malawi and WHO (2009) Malawi National STEPS Survey for Chronic Non-Communicable Diseases and their Risk Factors. Final Report Ministry of Health,Malawi.
- 51.Levitan EB, Song Y, Ford ES, Liu S (2004) Is nondiabetic hyperglycemia a risk factor for cardiovascular disease?: a meta-analysis of prospective studies. Archives of internal medicine 164: 2147–2155. doi: 10.1001/archinte.164.19.2147 [DOI] [PubMed] [Google Scholar]
- 52.Balkau B, Bertrais S, Ducimetiere P, Eschwege E (1999) Is there a glycemic threshold for mortality risk? Diabetes care 22: 696–699. [DOI] [PubMed] [Google Scholar]
- 53.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, et al. (2000) The 1997 American Diabetes Association and 1999 World Health Organization criteria for hyperglycemia in the diagnosis and prediction of diabetes. Diabetes care 23: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 54.Shaw JE, Zimmet PZ, Hodge AM, de Courten M, Dowse GK, et al. (2000) Impaired fasting glucose: how low should it go? Diabetes Care 23: 34–39. [DOI] [PubMed] [Google Scholar]
- 55.Garcez MR, Pereira JL, Fontanelli MdM, Marchioni DML, Fisberg RM (2014) Prevalence of dyslipidemia according to the nutritional status in a representative sample of Sao Paulo. Arquivos brasileiros de cardiologia 103: 476–484. doi: 10.5935/abc.20140156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joshi SR, Anjana RM, Deepa M, Pradeepa R, Bhansali A, et al. (2014) Prevalence of dyslipidemia in urban and rural India: the ICMR–INDIAB study. PloS one 9: e96808 doi: 10.1371/journal.pone.0096808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okafor CI (2012) The metabolic syndrome in Africa: Current trends. Indian journal of endocrinology and metabolism 16: 56 doi: 10.4103/2230-8210.91191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan BY, Kantilal HK, Singh R (2008) Prevalence of metabolic syndrome among Malaysians using the international diabetes federation, national cholesterol education program and modified World Health Organization definitions. Malaysian Journal of Nutrition 14: 65–77. [PubMed] [Google Scholar]
- 59.Brown CD, Higgins M, Donato KA, Rohde FC, Garrison R, et al. (2000) Body mass index and the prevalence of hypertension and dyslipidemia. Obesity 8: 605–619. [DOI] [PubMed] [Google Scholar]
- 60.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, et al. (2005) Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Jama 293: 1868–1874. doi: 10.1001/jama.293.15.1868 [DOI] [PubMed] [Google Scholar]
- 61.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, et al. (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116: 39–48. doi: 10.1161/CIRCULATIONAHA.106.675355 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOC)
(PDF)
Data Availability Statement
Summary data are available in S1 File. The National WHO Steps survey data underlying this study are National Data available from the Ethiopian Public Health Institute, Ethiopia. Interested, qualified researchers may submit queries related to data access to: Dr. Getachew Addis; Address: EPHI Scientific and EthicsReview Office, P.O Box 1242, Addis Ababa, Ethiopia; Office Phone: +251-118-685503/15; E-mail: get_ast@yahoo.com.