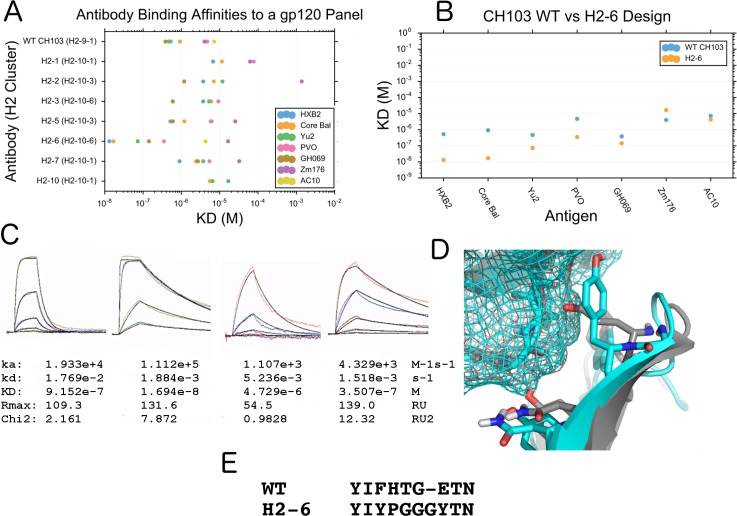
Fig 9. Binding of designed antibodies to HIV gp120.
(A) Apparent binding affinity (KD) of WT CH103 antibody and designed antibodies to a panel of gp120 antigens. Here, 30 designs were expressed and tested, where 7 had detectable binding to these gp120s. (B) Binding affinity (KD) of the designed antibody, H2-6, versus the wild-type antibody CH103. (C) Kinetic sensorgrams of CH103 WT and design H2-6 to two select GP120s, Core Bal and PVO as determined through a Biacore 4000. (D) Model of the interface changes in design H2-6, with designed H2 cluster H2-10-1 (cyan), superimposed onto the WT antibody from PDB ID 4JAN (gray) (E) Alignment of H2-6 and the WT antibody CH103 from PDB ID 4JAN.