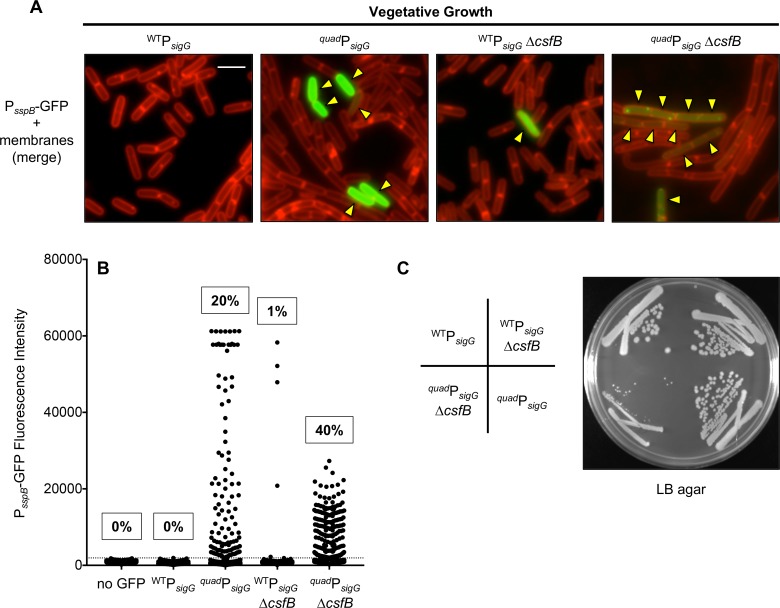
Fig 7. Misexpression of sigG causes ectopic σG activity in a subset of vegetative cells.
(A, B) GFP production from a σG-dependent PsspB-gfp reporter was monitored by fluorescence microscopy of vegetatively growing cells in which sigG was expressed from its wild type regulatory sequences (WTPsigG) or from regulatory sequences modified to remove or repair the four features identified in this study to dampen sigG expression (quadPsigG). Additionally, cells either harbored wild type csfB, or were deleted for the gene (ΔcsfB). (Strains EBM192 [WTPsigG], EBM276 [quadPsigG], EBM282 [WTPsigG ΔcsfB], and EBM287 [quadPsigG-sigG ΔcsfB].) In (A), representative microscopy images are shown with GFP fluorescence (false-colored green) merged with membrane fluorescence from the dye FM 4–64 (false-colored red). Yellow arrowheads indicate vegetative cells with visible GFP fluorescence. Scale bar 5 μm. In (B), GFP fluorescence intensity (with background subtracted) for more than 500 cells of each strain, including a “no GFP” control strain (PY79) lacking the PsspB-gfp reporter, is shown in column scatter graph format, with each cell represented by a black dot. Cells exhibiting fluorescence intensity above the cut off value (three standard deviations above mean auto-fluorescence of the “no GFP” strain; gray dashed line) were determined to have detectable σG activity. The percentage of cells with this activity is indicated above the respective strain. Note that ~60000 fluorescence units is the upper limit of detection under our microscopy settings. (C) Simultaneous deletion of csfB and misexpression of sigG from quadPsigG causes synthetic toxicity to vegetatively growing B. subtilis. The same strains described in (A) were struck onto LB agar and were photographed after ~18 hours of growth at 37°C.