Figure 3.
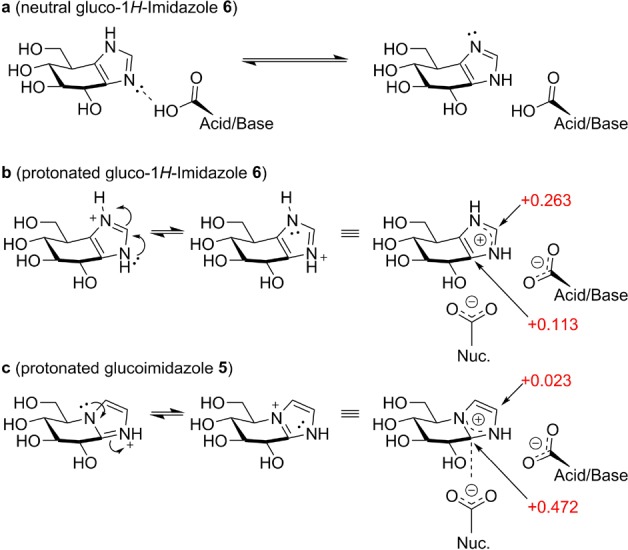
Interactions of gluco-1H-imidazole 6 and classical glucoimidazole 5 with the catalytic residues. (a) Prototropic tautomerism of 6. (b) Positive charge is delocalized onto the “apical” carbon in protonated 6. (c) In 5, positive charge is delocalized onto the anomeric equivalent carbon, ideally located for charge–charge interaction with the nucleophile residue. Mulliken charges are annotated in red.
