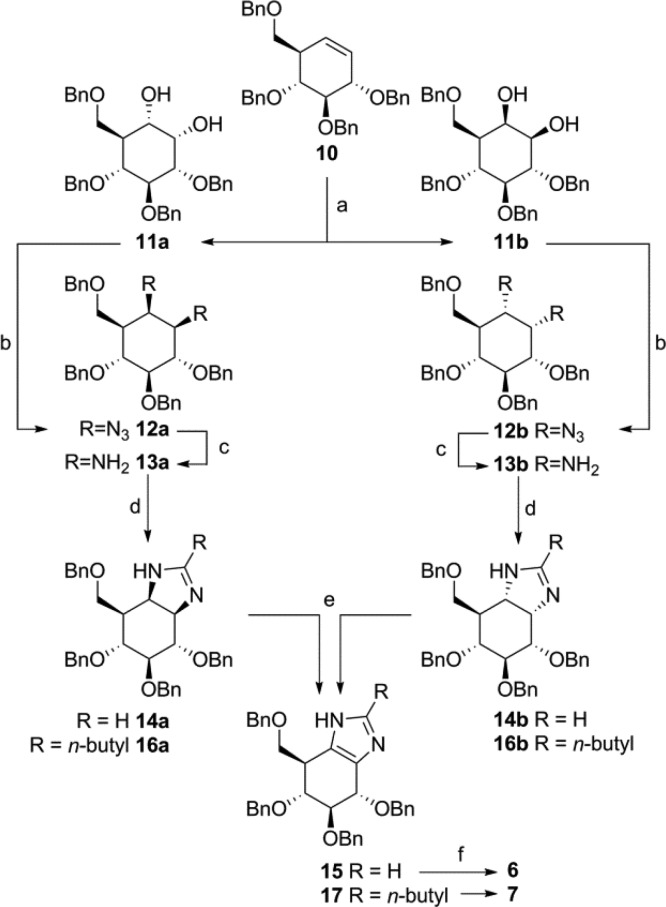
Scheme 1. Synthesis of 1H-Imidazoles 6 and 7.
Reagents and conditions: (a) RuCl3·3H2O (7 mol %), NaIO4, EtOAc, MeCN, H2O, 0 °C, 90 min, 11a 40%, 11b 32%; (b) MsCl, N-methyl-imidazole, Et3N, CHCl3, rt, 16 h, then NaN3, DMF, 100 °C, 16 h, 12a 74%, 12b 58%; (c) PtO2, H2, THF, rt, 16 h, 13a 80%, 13b 96%; (d) trimethyl orthoformate, HFIP, rt, 16 h, 14a 76%, 14b 87%; or trimethyl orthovalerate, HFIP, rt, 16 h, 16a 74%, 16b 78%; (e) for 15: IBX, DMSO, 45 °C, 16 h, 75% from 14a, 71% from 14b; for 17: (COCl)2, DMSO, DCM, −60 °C, 1 h, 76% from 16a; 70% from 16b; (f) Pd(OH)2/C, H2, HCl, MeOH, quant. 6; quant. 7.