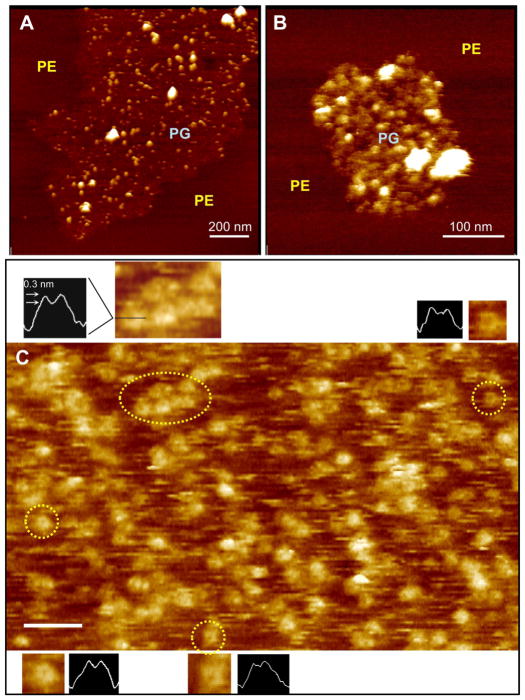
Figure 4.
HRF-AFM of untreated G93ASOD1 bound to PG membrane reveals tetramer-sized assemblies, in some cases, assuming “open” channel-like conformations. (A,B) Lipid raft-like PG subdomains surrounded by PE with selectively bound untreated G93ASOD1 molecules of a size consistent with tetrameric structuring (12–15 nm). The membrane-bound G93ASOD1 oligomers (diameter of smaller white bumps 13.5 nm ± 0.4, n=20) co-adsorbed with larger aggregates (larger brighter structures) bound only to the PG subdomains. (C) High magnification HRF-AFM scan reveals tetramer-sized G93ASOD1 bound to DOPG bilayer in aqueous buffer. The locations representing four pairs of figure insets enlarged to show more detail, including cross sectional line traces through the centers of each structure (dotted yellow circles, insets, and black horizontal line in top left inset). Note the small circular darkened areas or depressions at the centers of many of the tetramer-sized structures (insets; approximately 5–8 nm across, 0.3 nm deep) indicating “open” channel-like structures that have inserted into the membrane. Full scale z-axis, 5 nm. Bar, 50 nm.