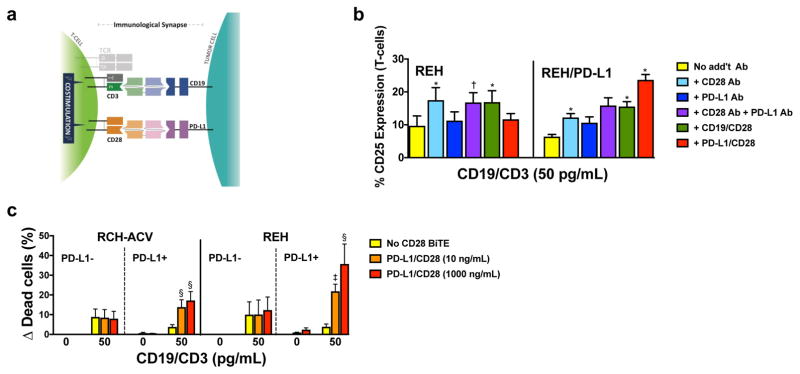
Figure 2. PD-L1/CD28 BiTE can reverse checkpoint inhibition into T-cell activation to overcome BiTE resistance.
(a) Scheme of co-activation of T-cells conferred by a PD-L1/CD28 BiTE and a paired CD3-directed BiTE recognizing another antigen on a PD-L1-expressing cancer cell. (b) Parental (PD-L1-negative) REH cells and PD-L1-transduced REH cells incubated with a CD19/CD3 BiTE (blinatumomab; 50 pg/mL) without additional antibody or with a CD28 antibody, a PD-L1 antibody, a CD19/CD28 BiTE, or a PD-L1/CD28 BiTE together with T-cells at an E:T ratio of 1:1 as indicated. After 24 hours, T-cell activation was quantified by flow cytometry via determination of cell surface expression of CD25. Results (mean ± SEM) are shown from 3 independent experiments. (c) Parental CD19+ lymphoid RCH-ACV and REH and sublines transduced to express PD-L1 were incubated with T-cells at an E:T ratio of 1:1 either alone or in the presence of a CD19/CD3 BiTE (blinatumomab; 50 pg/mL) with or without various concentrations of a PD-L1/CD28 BiTE as indicated. After 48 hours, drug-induced cytotoxicity was determined by flow cytometry. Increases in the percentage of DAPI-positive cells in BiTE-treated cells are compared with corresponding cells that were incubated without antibody, and results are shown as mean ± SEM from 3 independent experiments performed in duplicate wells. Throughout, two-sided P values were calculated using repeated measure one-way or two-way ANOVA with Tukey’s multiple comparison test as appropriate. For all panels, *P<0.05, †P<0.01, ‡P<0.001, and §P<0.0001 vs. corresponding control.