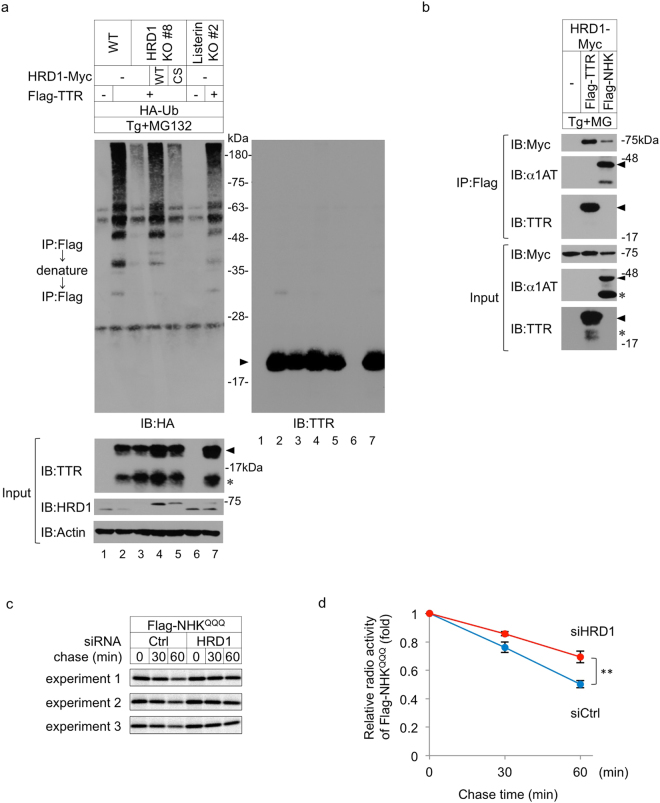
Figure 3.
HRD1 is required for the ubiquitination of ERpQC substrates. (a) HRD1 contributes to the ubiquitination of ERpQC substrates through its E3 ligase activity. WT, HRD1 KO (clone #8) or Listerin KO (clone #2) HEK293 cells were treated with 50 nM Tg and 200 nM MG132 for 16 h after transfection with the indicated combinations. Flag-TTR-Myc was immunoprecipitated with an anti-Flag Ab affinity gel. After incubation with the denaturing buffer containing 1% SDS, Flag-TTR-Myc was re-immunoprecipitated with an anti-Flag Ab affinity gel and analyzed by IB with the indicated Abs. Arrowheads and asterisk indicate signal peptide-uncleaved TTR (STTR) and signal peptide-cleaved TTR, respectively. CS, HRD1 (C291S/C329S)-Myc-His; Flag-TTR, Flag-TTR-Myc; HA-Ub, HA-Ubiquitin. (b) HRD1 interacts with ERpQC substrates. HEK293 cells were transfected with HRD1-Myc-His and Flag-TTR-HA or Flag-NHKQQQ-HA and treated with 50 nM Tg and 200 nM MG132. Cell lysates were analyzed by IP-IB using the indicated Abs. Arrowheads and asterisks indicate ERpQC substrates (STTR and SNHKQQQ) and ER translocated proteins (signal peptide-cleaved TTR and NHKQQQ), respectively. (c and d) The requirement of HRD1 for the degradation of ERpQC substrates. HEK293 cells were transfected with siRNA against Ctrl or HRD1 and Flag-NHKQQQ-HA and treated with 50 nM Tg for 16 h. Cells were pulse-labeled with [35S]-methionine/cysteine for 15 min and chased for the indicated time periods. Flag-NHKQQQ-HA was immunoprecipitated with an anti-Flag Ab affinity gel and analyzed by SDS-PAGE and autoradiography (c). The relative radioactivities in SNHKQQQ at different times of chase were calculated and shown as fold decreases relative to the intensity observed at 0 h chase. Values are expressed as the mean ± S.D. (**) P < 0.01; significance calculated by Student’s t-test (n = 3). (a–c) Full-length blots and gels are presented in Supplementary Fig. 5.