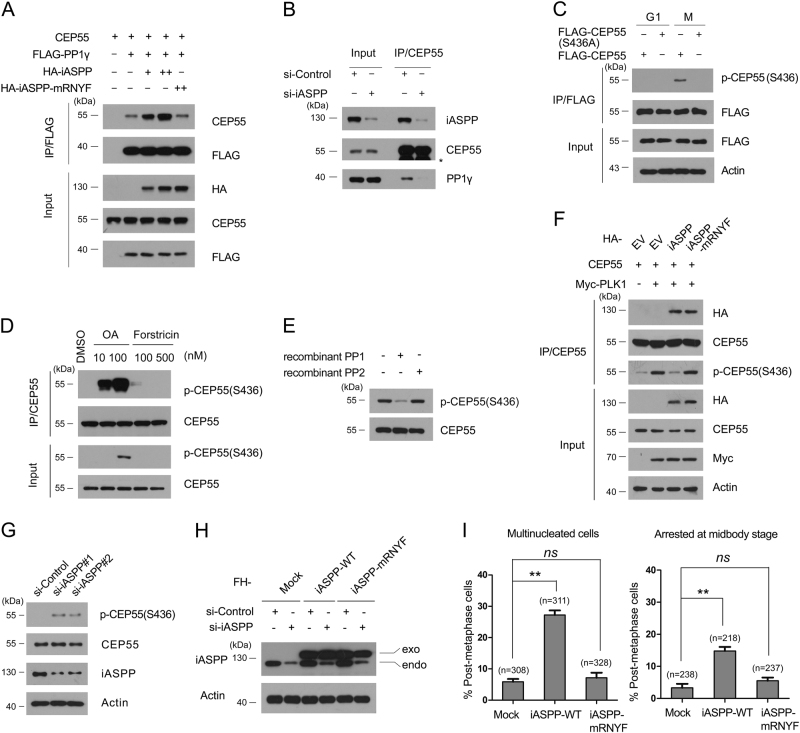
Fig. 5. iASPP–PP1 complex dephosphorylates mitotic CEP55 at Ser436.
a iASPP facilitates the interaction between CEP55 and PP1γ in a PP1-binding-dependent manner. 293T cells were co-transfected with indicated constructs. After 24 h, cell lysates were prepared for immunoprecipitation with anti-FLAG antibody and WB analyses using indicated antibodies. b iASPP depletion reduces the endogenous interaction between CEP55 and PP1γ. HeLa cells were transfected with control or iASPP siRNAs. After 48 h, cell lysates were prepared for immunoprecipitation with anti-CEP55 antibody and WB analyses using indicated antibodies. The asterisk (*) denotes a non-specific band. c Validation of phospho-CEP55 (S436) antibody. HeLa cells were transfected with FLAG-CEP55 or CEP55 S435A mutant constructs. After 48 h, cell lysates were prepared for immunoprecipitation with anti-FLAG antibody and WB analyses using indicated antibodies. d PP1, but not the PP2 inhibitor increases phospho-CEP55 (S436) signal. HeLa cells were treated with different doses of Okadaic acid (OA) or Fostriecin for 12 h. Cell lysates were prepared for immunoprecipitation with the anti-CEP55 antibody and detected by WB analyses using the indicated antibodies. e PP1 dephosphorylates CEP55 at Ser436 in vitro. HeLa cells were arrested in prometaphase by a sequential thymidine-nocodazole block, then endogenous CEP55 was immunoprecipitated and incubated with the recombinant PP1 or PP2. The phospho-CEP55 (S436) signal was analyzed by WB using the indicated antibodies. f iASPP antagonizes PLK1-mediated CEP55 (S436) phosphorylation. 293T cells were transfected with pcDNA3-CEP55、FLAG-PP1γ and different dose of HA-iASPP (WT or mRNYF) constructs. After 24 h, cell lysates were prepared for immunoprecipitation with anti-FLAG antibody and WB using indicated antibodies. g iASPP depletion increases phospho-CEP55 (S436) signal. HeLa cells were transfected with control or iASPP siRNA. After 48 h, cell lysates were prepared for WB analyses with indicated antibodies. h WB analyses of iASPP proteins following siRNA treatment in HeLa cells stably expressing a FH-iASPP constructs resistant to the siRNAs targeting endogenous iASPP. i Stably expression of siRNA-resistant iASPP, but not mRNYF, in HeLa cells rescued cytokinesis defects caused by iASPP depletion. All data shown are mean values ± SD (error bar) from three replicates