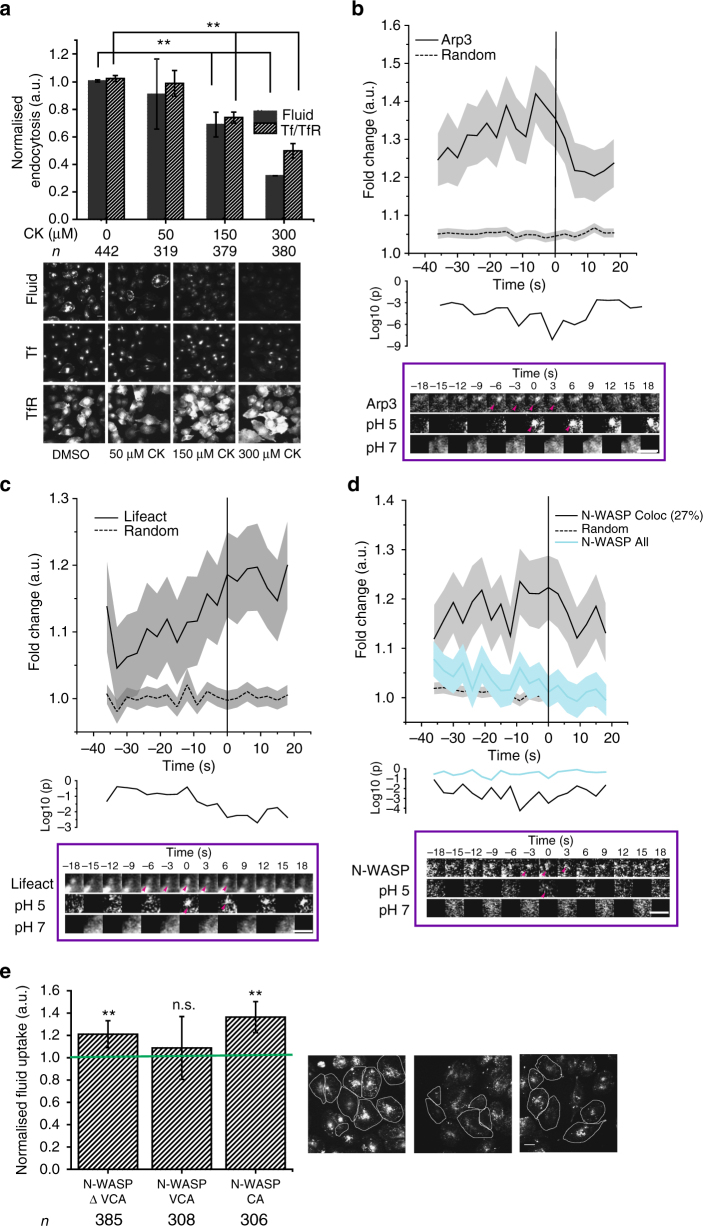
Fig. 6.
Arp2/3-based actin machinery is required for CG endocytosis. a Histograms (top) show quantification of fluid phase and TfR uptake in AGS cells treated with DMSO alone (0 µM) or the indicated concentrations of ARP2/3 inhibitor, CK666, normalised to DMSO-treated controls, along with its representative images (below). Data are pooled from two independent experiments and the number of cells shown indicated the graph. b–d Graphs show the average normalised fluorescence intensity vs. time traces for the recruitment of mCherry-ARP3 (b), pRuby-Lifeact (c) and mCherry-NWASP (d) to the forming SecGFP-GPI endocytic sites, and its corresponding random intensity trace (n, Table 1). The random traces were derived from randomly assigned spots of the same radius as the endocytic regions, as detailed in S.I. Endocytic distribution at each time point was compared to the random distribution by Mann–Whitney U test and the log10 (p) [log10 (0.05) is −1.3 and log10 (0.001) is −2.5] is plotted below each trace (b–d). Representative montages are depicted below the graphs. Arrowheads indicate the newly formed endocytic vesicle. e Histogram (left) shows normalised 5-min mean fluid-phase uptake in AGS cells overexpressing pIRES-CA domain, GFP-VCA domain and GFP-N-WASP∆VCA from N-WASP compared to un-transfected cells and representative images (right). The transfected cells are outlined. Data were pooled from two independent experiments and the number of cells shown below the graph. Error bars represent s.e.m. (b–d) and s.d. (a, e). p value <0.01 (*), and 0.001(**) by Mann–Whitney U test (a, e). Scale bar, 1.5 µm (b–d), 20 µm (a, e)