Abstract
Effective treatments for patients with castration‐resistant prostate cancer (CRPC) have not yet been established. Novel approaches for identification of putative therapeutic targets for CRPC are needed. Analyses of RNA sequencing of microRNA (miRNA) expression revealed that miR‐99a‐3p (passenger strand) is significantly downregulated in several types of cancers. Here, we aimed to identify novel miR‐99a‐3p regulatory networks and therapeutic targets for CRPC. Ectopic expression of miR‐99a‐3p significantly inhibited cancer cell proliferation, migration, and invasion in PCa cells. Non‐SMC condensin I complex subunit G (NCAPG) was a direct target of miR‐99a‐3p in PCa cells. Overexpression of NCAPG was detected in CRPC clinical specimens and was significantly associated with shorter disease‐free survival and advanced clinical stage. Knockdown of NCAPG inhibited cancer cell aggressiveness. The passenger strand miR‐99a‐3p acted as an antitumor miRNA in naïve PCa and CRPC. NCAPG was regulated by miR‐99a‐3p, and its overexpression was involved in CRPC pathogenesis. Involvement of passenger strand of miRNA in cancer pathogenesis is novel concept, and identification of antitumor miRNA regulatory networks in CRPC might be provided novel prognostic markers and therapeutic targets for this disease.
Keywords: Castration‐resistant prostate cancer, microRNA, miR‐99a‐3p, miR‐99a‐5p, non‐SMC condensin I complex subunit G
Introduction
In developed countries, prostate cancer (PCa) is one of the most commonly diagnosed cancers, identified by prostate‐specific antigen (PSA) screening; PCa is also the third leading cause of cancer‐related death among men 1. Most naïve PCa initially responds well to androgen‐deprivation therapy (ADT). However, during ADT treatment, PCa cells acquire ADT treatment resistance and progress to a lethal pathology known as castration‐resistant prostate cancer (CRPC) 2. Cancer cells that have reached CRPC can cause distant metastasis, and effective treatments for patients with CRPC have not yet been established 3. Identification of the molecular pathogenesis underlying acquisition of androgen‐independent and metastatic signaling pathways based on advanced genomic approaches is essential for further understanding of this disease.
MicroRNAs (miRNAs) are endogenous small RNAs (molecules 18–23 bases in length) that act as central players regulating the expression control of protein‐coding and protein‐noncoding RNAs 4, 5. Interestingly, a single miRNA can directly regulate a vast number of RNAs in human cells 6. Therefore, aberrant expression of miRNAs can disrupt normal control of RNA expression in cancer cells. Furthermore, dysregulation of miRNAs is contributed to cancer cell malignancies, such as progression, metastasis, and treatment resistance 7, 8, 9, 10.
Analysis of our original miRNA expression signatures of cancers based on RNA sequencing revealed that several passenger strands of miRNAs, for example, miR‐145‐3p, miR‐150‐3p, miR‐149‐3p, miR‐199a‐3p, and miR‐144‐5p, are downregulated in several cancer tissues and act as antitumor miRNAs in cancer cells 11, 12, 13, 14, 15. However, this is inconsistent with the paradigm that the guide strand of miRNA is loaded into the miRNA‐induced silencing complex (RISC) and represses translation or degradation of target genes 16, whereas the passenger strand of miRNA is thought to be destroyed in the cytoplasm and to have no function 17, 18, 19.
We have sequentially identified the functional significance of passenger strands of miRNAs in cancer cells based on miRNA signatures 11, 12, 13, 14, 15. In this study, we focused on miR‐99a‐5p (guide strand) whose expression was significantly downregulated in our miRNA signature of metastatic CRPC 15 and investigated the functional roles including passenger strand miR‐99a‐3p in naïve PCa and CRPC cells. Previous studies have shown that the guide strand miR‐99a‐5p has antitumor roles in several cancers 20, 21, 22, 23. In contrast, no studies have reported the role of the passenger strand miR‐99a‐3p in cancer cells. Novel strategies based on passenger strands of miRNAs will enhance our understanding of the molecular pathways underlying naïve PCa and CRPC pathogenesis.
Materials and Methods
Collection of clinical prostate specimens and cell lines
Clinical specimens were collected at Teikyo University Chiba Medical Center and Chiba University Hospital from 2013 to 2016. Patient characteristics and clinical features are summarized in Table 1. The protocol of this study was approved by the Institutional Review Boards of Teikyo University and Chiba University. We have experimented with human PCa cell lines (PC3, DU145, and C4‐2). The cells were maintained as previously reported 11, 15, 24, 25.
Table 1.
Patient characteristics
| Patient No. | Procedure | Diagnosis | Age (years) | PSA (ng/mL) | Gleason score | T | N | M | Stage | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Biopsy | Non‐PCa | 57 | 5.71 | – | – | – | – | – | RT‐PCR |
| 2 | Biopsy | Non‐PCa | 74 | 9.45 | – | – | – | – | – | RT‐PCR |
| 3 | Biopsy | Non‐PCa | 70 | 8.58 | – | – | – | – | – | RT‐PCR |
| 4 | Biopsy | Non‐PCa | 73 | 4.8 | – | – | – | – | – | RT‐PCR |
| 5 | Biopsy | Non‐PCa | 67 | 6.91 | – | – | – | – | – | RT‐PCR |
| 6 | Biopsy | Non‐PCa | 50 | 7.05 | – | – | – | – | – | RT‐PCR |
| 7 | Biopsy | Non‐PCa | 74 | 9.91 | – | – | – | – | – | RT‐PCR |
| 8 | Biopsy | Non‐PCa | 76 | 20.9 | – | – | – | – | – | RT‐PCR |
| 9 | Biopsy | Non‐PCa | 59 | 4.5 | – | – | – | – | – | RT‐PCR |
| 10 | Biopsy | Non‐PCa | 75 | 1.1 | – | – | – | – | – | RT‐PCR |
| 11 | Biopsy | Non‐PCa | 60 | 7.29 | – | – | – | – | – | RT‐PCR |
| 12 | Biopsy | Non‐PCa | 73 | 38.7 | – | – | – | – | – | RT‐PCR |
| 13 | Biopsy | Non‐PCa | 69 | 11.9 | – | – | – | – | – | RT‐PCR |
| 14 | Biopsy | Non‐PCa | 77 | 23.3 | – | – | – | – | – | RT‐PCR |
| 15 | Biopsy | Non‐PCa | 61 | 4.57 | – | – | – | – | – | RT‐PCR |
| 16 | Biopsy | Non‐PCa | 59 | 7.37 | – | – | – | – | – | RT‐PCR |
| 17 | Biopsy | Non‐PCa | 65 | 5.06 | – | – | – | – | – | RT‐PCR |
| 18 | Biopsy | HSPC | 70 | 75.7 | 4 + 5 | 4 | 1 | 1 | IV | RT‐PCR |
| 19 | Biopsy | HSPC | 78 | 1800 | 4 + 5 | 4 | 1 | 1 | IV | RT‐PCR |
| 20 | Biopsy | HSPC | 75 | 68.4 | 5 + 4 | 4 | 1 | 0 | IV | RT‐PCR |
| 21 | Biopsy | HSPC | 62 | 38.7 | 4 + 5 | 2b | 1 | 0 | IV | RT‐PCR |
| 22 | Biopsy | HSPC | 70 | 25.5 | 4 + 5 | 3b | 0 | 0 | III | RT‐PCR |
| 23 | Biopsy | HSPC | 88 | 888 | 4 + 5 | 3b | 1 | 1 | IV | RT‐PCR |
| 24 | Biopsy | HSPC | 69 | 33.9 | 4 + 5 | 4 | 0 | 1 | IV | RT‐PCR |
| 25 | Biopsy | HSPC | 62 | 62.3 | 4 + 5 | 3b | 1 | 0 | IV | RT‐PCR |
| 26 | Biopsy | HSPC | 78 | 5 | 4 + 5 | 2c | 0 | 1b | IV | RT‐PCR |
| 27 | Biopsy | HSPC | 64 | 449 | 4 + 5 | 3b | 1 | 1 | IV | RT‐PCR |
| 28 | Biopsy | HSPC | 81 | 365 | 4 + 5 | 4 | 1 | 1 | IV | RT‐PCR |
| 29 | Biopsy | HSPC | 76 | 715 | 5 + 4 | 4 | 1 | 1 | IV | RT‐PCR |
| 30 | Biopsy | HSPC | 79 | 555 | 4 + 5 | 3 | 1 | 1 | IV | RT‐PCR |
| 31 | Biopsy | HSPC | 63 | 1120 | 4 + 5 | 2c | 0 | 1b | IV | RT‐PCR |
| 32 | Biopsy | HSPC | 67 | 4.95 | 4 + 5 | 4 | 1 | 1b | IV | RT‐PCR |
| 33 | Biopsy | HSPC | 70 | 19.5 | 5 + 5 | 4 | 1 | 1c | IV | RT‐PCR |
| 34 | Biopsy | CRPC | 69 | 15.8 | 5 + 4 | 3b | 1 | 1 | IV | RT‐PCR |
| 35 | Biopsy | CRPC | 72 | 212 | 5 + 4 | 4 | 1 | 1 | IV | RT‐PCR |
| 36 | Biopsy | CRPC | 71 | 4.4 | 4 + 5 | 4 | 1 | 1 | IV | RT‐PCR |
| 37 | Biopsy | CRPC | 68 | 7.54 | 4 + 5 | 4 | 1 | 1b | IV | RT‐PCR |
| 38 | Prostatectomy | HSPC | 65 | 5.3 | 4 + 5 | 2a | 0 | 0 | II | IHC |
| 39 | Prostatectomy | HSPC | 61 | 21.48 | 4 + 4 | 3a | 0 | 0 | III | IHC |
| 40 | Autopsy | CRPC | 64 | 4100 | 4 + 5 | 4 | 1 | 1c | IV | IHC |
| 41 | Autopsy | CRPC | 75 | 4690 | 4 + 5 | 4 | 1 | 1c | IV | IHC |
Quantitative real‐time reverse transcription polymerase chain reaction (qRT‐PCR)
The procedure of PCR quantification is described in our previous reports 11, 15, 24, 25, 26. Expression levels of miR‐99a‐5p and miR‐99a‐3p normalized to expression of RNU48 were analyzed by TaqMan qRT‐PCR. The expression levels of NCAPG and pri‐miR‐99a were assessed by being normalized with GAPDH or GUSB. Detailed product numbers of reagents used are shown in the Table S1.
Transfection with mature miRNA, small‐interfering RNA (siRNA), or plasmid vectors
We used the mature miRNAs, siRNAs, and plasmid vectors described below: Pre‐miR miRNA precursor (hsa‐miR‐99a‐5p; assay ID: PM10719 and hsa‐miR‐99a‐3p; assay ID: PM12983; Applied Biosystems, Foster City, CA), Stealth Select RNAi siRNAs; si‐NCAPG (cat. nos. HSS127430 and HSS184671; Invitrogen, Carlsbad, CA), and negative control miRNA/siRNA (P/N: AM17111; Applied Biosystems). RNAs were incubated with OPTI‐MEM (Invitrogen) and Lipofectamine RNAiMax reagent (Invitrogen) at a concentration of 10 nmol/L by reverse transfection. We used NCAPG plasmid vector designed by ORIGENE (cat. no. SC111395; Rockville, MD). Transfection procedures were described as previous studies 11, 15, 24, 25, 26.
Cell proliferation, migration, and invasion assays
As functional analyses, cell proliferation, migration, and invasion assays were carried out based on our past reports 11, 15, 24, 25, 26. We confirmed all experiments in triplicate.
Confirmation of miRNAs incorporated into the RNA‐induced silencing complex (RISC) by Ago2 immunoprecipitation
To investigate whether exogenous miR‐99a‐5p and miR‐99a‐3p were incorporated into the RISC, we carried out immunoprecipitation assays using a microRNA isolation kit for human Ago2 (Wako, Osaka, Japan). The procedure is described in our past reports 11, 15.
Identification strategy of estimated target genes regulated by miR‐99a‐3p in PCa cells
To identify putative miR‐99a‐3p target genes, we used in silico database analyses and comprehensive gene expression analyses by microarray technologies, as described previously 11, 15, 24, 25, 26. The microarray data were deposited into the GEO database (https://www.ncbi.nlm.nih.gov/geo/; accession number: GSE85614).
Western blotting
Immunoblotting was carried out with rabbit anti‐NCAPG antibodies (1:750; ab56382; Abcam, Cambridge, UK). We used antiglyceraldehyde 3‐phosphate dehydrogenase (GAPDH) antibodies (1:10000, ab8245; Abcam) for an internal loading control. The experimental procedures were performed as described in our past reports 11, 24, 25, 26.
Plasmid construction and dual‐luciferase reporter assays
A partial wild‐type sequence of the NCAPG 3′‐untranslated region (UTR) or a sequence having a deletion of the miR‐99a‐3p target site was inserted into the psiCHECK‐2 vector (C8021; Promega, Madison, WI). The procedures were reported previously 11, 24, 25, 26.
Immunohistochemistry
Tissue specimens were incubated overnight at 4°C with anti‐NCAPG antibodies (1:150; ab56382; Abcam). The procedures were described previously 11, 15, 24, 25, 26.
The Cancer Genome Atlas (TCGA) database analyses of PCa
To identify the clinical significance of NCAPG, we applied to TCGA database. The gene expression and clinical data were analyzed using cBioportal (http://www.cbioportal.org/) 27. The data were obtained on 30 May 2017.
Statistical analysis
The relationship between the two groups was analyzed using the Mann–Whitney U test. The relationship of three variables or more was analyzed using Bonferroni‐adjusted Mann–Whitney U tests. The correlation between two groups was evaluated by Spearman's rank test. Survival analyses by Kaplan–Meier method and log‐rank test was performed using JMP software (version 13; SAS Institute Inc., Cary, NC). For all other analyses, Expert StatView (version 5, SAS Institute Inc.) was used.
Results
Expression levels of miR‐99a‐5p and miR‐99a‐3p in PCa specimens and cell lines
In human genome, miR‐99a is located on chromosome 21q21.1 and the mature sequences of miR‐99a‐5p and miR‐99a‐3p are 5′‐AACCCGUAGAUCCGAUCUUGUG‐3′and 5′‐CAAGCUCGCUUCUAUGGGUCUG‐3′, respectively (Fig. S1). We validated the expression levels of miR‐99a‐5p and miR‐99a‐3p in PCa tissues (hormone‐sensitive prostate cancer [HSPC]: n = 16, CRPC: n = 4), normal tissues (n = 17), and PCa cell lines (PC3, DU145, and C4‐2). Table 1 shows the patients’ characteristics. The expression levels of miR‐99a‐5p and miR‐99a‐3p were markedly lower in PCa and CRPC tissues than in normal tissues (miR‐99a‐5p: P = 0.0001 and P < 0.0001, miR‐99a‐3p: P = 0.0047 and P = 0.0001; Fig. 1A and B). miR‐99a‐5p and miR‐99a‐3p were expressed with positive correlation. (r = 0.771, P < 0.0001; Fig. 1C). Furthermore, the expression level of pri‐miR‐99a, a precursor of miR‐99a‐5p/‐3p, was also examined and the expression was downregulated in the PCa tissues (Fig. S2).
Figure 1.
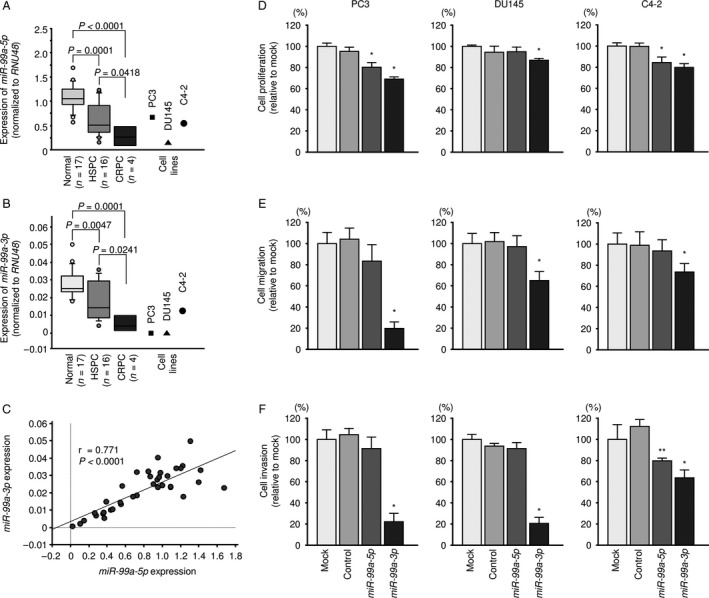
Expression of miR‐99a‐5p/3p in clinical prostate specimens and functional analysis of miR‐99a‐5p/3p in PCa cell lines. (A) Expression levels of miR‐99a‐5p in PCa clinical specimens and cell lines determined using qRT‐PCR. RNU48 was used as an internal control. (B) Expression levels of miR‐99a‐3p in PCa clinical specimens and cell lines. (C) Correlations among the relative expression levels of miR‐99a‐5p and miR‐99a‐3p. (D‐F) Cell proliferation, migration, and invasion assays in cells transfected with miR‐99a‐5p/3p. *P < 0.0001 and **P < 0.001.
Both miR‐99a‐5p and miR‐99a‐3p bound to Ago2
To verify that both miR‐99a‐5p and miR‐99a‐3p functioned by incorporation into the RISC, we performed immunoprecipitation with antibodies targeting Ago2 which plays a key role of RISC (Fig. S3A). Quantification of miRNAs bound to Ago2 was detected by PCR methods. The amount of miR‐99a‐5p bound to Ago2 was remarkably higher than that in cells transfected with mock, miR‐control, and miR‐99a‐3p (P < 0.0001; Fig. S3B). Similarly, the amount of miR‐99a‐3p bound to Ago2 was markedly higher than that in cells transfected with mock, miR‐control, and miR‐99a‐5p (P < 0.0001; Fig. S3B).
Effects of restoring miR‐99a‐5p/3p on cell proliferation, migration, and invasion activities in PCa cell lines
To confirm the tumor‐suppressive roles of miR‐99a‐5p and miR‐99a‐3p, we carried out ectopic expression assays by miRNA transfection into PC3, DU145, and C4‐2 cells. According to the results of functional assays, cancer cell proliferation, migration activity, and invasion activity were all remarkably inhibited by transfection with miR‐99a‐3p compared with those of mock‐ or miR‐control‐transfected PC3, DU145 C4‐2 cells (P < 0.0001, P < 0.0001, and P < 0.0001, respectively; Fig. 1D–F, S4A and B). Cell proliferation assay was also performed in LNCaP cells, and its ability was suppressed by transfection with miR‐99a‐3p (data not shown). In contrast, miR‐99a‐5p showed no significant antitumor effects (Fig. 1D‐F).
Search for putative oncogenes regulated by miR‐99a‐3p in PCa cells
We focused on miR‐99a‐3p, which showed marked antitumor effects. The selection strategy of miR‐99a‐3p target genes is shown in Figure 2A. Initially, we used the TargetScan Human 7.1 database and found that 1591 genes had theoretical target sites for miR‐99a‐3p in their 3′‐UTRs. Next, we extracted genes whose expression levels were decreased by transfection with miR‐99a‐3p by gene expression analysis (GEO accession number: GSE85614). Genes that were markedly decreased by transfection into PC3 cells with miR‐99a‐3p are shown in Table 2 (fold‐change log2 < −2.0). In this study, a total of 30 putative oncogenic targets of miR‐99a‐3p regulation were identified in PC cells. We investigated further whether it has related to the pathogenesis of PCa and these targets using TCGA database. Among these targets, 17 genes (NCAPG, SGOL1, RRM2, ESCO2, ZNF695, CDK1, NEK2, FANCI, FAM64A, ZWINT, PIGL, KIF11, MCM4, BRCA1, CDKN3, GRIA2, and MKI67) were involved in PCa pathogenesis, high expression of these genes were significantly associated with disease‐free survival rate (Figs 2B, 3).
Figure 2.
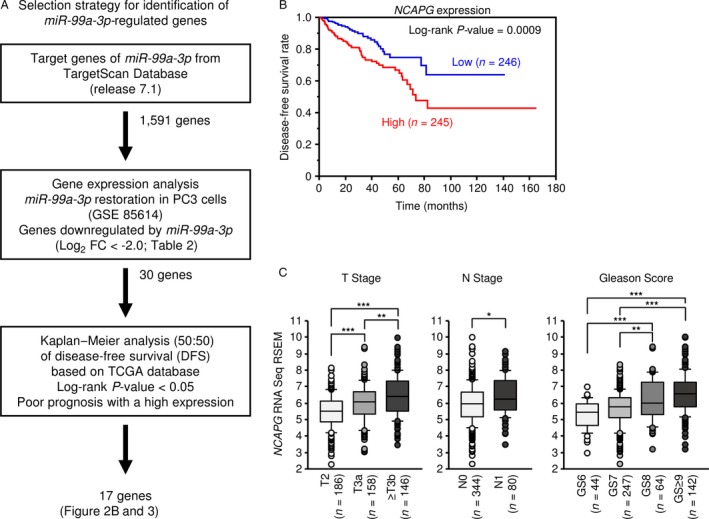
Identification of miR‐99a‐3p target genes and relationship between NCAPG and clinicopathological factors. (A) Flowchart of the strategy for identification of miR‐99a‐3p target genes. (B) Kaplan–Meier patient survival curves for disease‐free survival rates based on NCAPG expression in patients with PCa from TCGA database. (C) According to TCGA database, the expression levels of NCAPG were significantly increased in cases of advanced T stage, advanced N stage, and high Gleason score. *P < 0.01, **P < 0.001, and ***P < 0.0001.
Table 2.
Putative target genes regulated by miR‐99a‐3p in PCa cells
| Entrez Gene ID | Gene symbol | Gene name | Location | Number of miR‐99a‐3p target sites | PC3 miR‐99a‐3p transfectant (Log2 ratio) |
|---|---|---|---|---|---|
| 64151 | NCAPG | Non‐SMC condensin I complex, subunit G | 4p15.31 | 1 | −3.87 |
| 151648 | SGOL1 | Shugoshin‐like 1 (S. pombe) | 3p24.3 | 1 | −3.49 |
| 6241 | RRM2 | Ribonucleotide reductase M2 | 2p25.1 | 1 | −3.39 |
| 157570 | ESCO2 | Establishment of sister chromatid cohesion N‐acetyltransferase 2 | 8p21.1 | 1 | −3.26 |
| 57116 | ZNF695 | Zinc finger protein 695 | 1q44 | 1 | −3.21 |
| 113115 | MTFR2 | Mitochondrial fission regulator 2 | 6q23.3 | 1 | −3.19 |
| 983 | CDK1 | Cyclin‐dependent kinase 1 | 10q21.2 | 1 | −3.03 |
| 4751 | NEK2 | NIMA‐related kinase 2 | 1q32.3 | 1 | −2.82 |
| 8693 | GALNT4 | UDP‐N‐acetyl‐alpha‐D‐galactosamine:polypeptide N‐acetylgalactosaminyltransferase 4 (GalNAc‐T4) | 12q21.33 | 2 | −2.72 |
| 143686 | SESN3 | Sestrin 3 | 11q21 | 1 | −2.61 |
| 55215 | FANCI | Fanconi anemia, complementation group I | 15q26.1 | 1 | −2.57 |
| 5557 | PRIM1 | Primase, DNA, polypeptide 1 (49 kDa) | 12q13.3 | 1 | −2.56 |
| 54478 | FAM64A | Family with sequence similarity 64, member A | 17p13.2 | 1 | −2.56 |
| 2218 | FKTN | Fukutin | 9q31.2 | 2 | −2.53 |
| 51522 | TMEM14C | Transmembrane protein 14C | 6p24.2 | 1 | −2.50 |
| 11130 | ZWINT | ZW10 interacting kinetochore protein | 10q21.1 | 1 | −2.47 |
| 9487 | PIGL | Phosphatidylinositol glycan anchor biosynthesis, class L | 17p11.2 | 1 | −2.47 |
| 3832 | KIF11 | Kinesin family member 11 | 10q23.33 | 1 | −2.43 |
| 4173 | MCM4 | Minichromosome maintenance complex component 4 | 8q11.21 | 1 | −2.42 |
| 672 | BRCA1 | Breast cancer 1, early onset | 17q21.31 | 1 | −2.40 |
| 586 | BCAT1 | Branched chain amino‐acid transaminase 1, cytosolic | 12p12.1 | 3 | −2.38 |
| 1033 | CDKN3 | Cyclin‐dependent kinase inhibitor 3 | 14q22.2 | 1 | −2.37 |
| 79917 | MAGIX | MAGI family member, X‐linked | Xp11.23 | 1 | −2.36 |
| 57082 | CASC5 | Cancer susceptibility candidate 5 | 15q15.1 | 1 | −2.35 |
| 2891 | GRIA2 | Glutamate receptor, ionotropic, AMPA 2 | 4q32.1 | 1 | −2.30 |
| 4288 | MKI67 | Antigen identified by monoclonal antibody Ki‐67 | 10q26.2 | 1 | −2.25 |
| 283487 | LINC00346 | Long intergenic non‐protein coding RNA 346 | 13q34 | 1 | −2.23 |
| 56952 | PRTFDC1 | Phosphoribosyl transferase domain containing 1 | 10p12.1 | 1 | −2.12 |
| 5140 | PDE3B | Phosphodiesterase 3B, cGMP‐inhibited | 11p15.2 | 1 | −2.04 |
| 2177 | FANCD2 | Fanconi anemia, complementation group D2 | 3p25.3 | 1 | −2.01 |
Figure 3.

Kaplan–Meier survival curves based on expression of 16 genes, excluding NCAPG, in patients with PCa. Kaplan–Meier patient survival curves for disease‐free survival rates based on expression of 16 genes, excluding NCAPG, in patients with PCa, according to TCGA database.
Finally, we focused on NCAPG, which showed the greatest reduction in expression following transfection with miR‐99a‐3p.
Clinical significance of NCAPG in PCa
According to TCGA database, NCAPG expression levels were closely related to prognosis and clinical stage in patients with PCa. High NCAPG expression group had remarkably shorter disease‐free survival (DFS) than that of the low expression group in patients with PCa (P = 0.0009, Fig. 2B). Moreover, the expression levels of NCAPG were markedly increased in cases with advanced T stage, advanced N stage, and high Gleason Score (Fig. 2C). These results indicated that NCAPG may affect disease progression and malignancy in PCa.
NCAPG was directly regulated by miR‐99a‐3p in PCa cells
The expression of NCAPG mRNA was significantly decreased by miR‐99a‐3p transfection compared to that of mock‐ or miR‐control‐transfected cells (Fig. 4A). Consistent with this, NCAPG protein expression was reduced by miR‐99a‐3p transfection (Fig. 4B).
Figure 4.
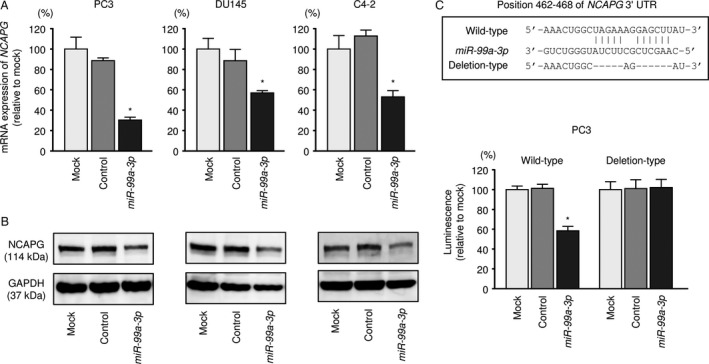
Direct regulation of NCAPG by miR‐99a‐3p in PCa cells. (A) NCAPG mRNA expression was evaluated using qRT‐PCR in PC3, DU145, and C4‐2 cells 48 h after transfection with miR‐99a‐3p. GAPDH was used as an internal control. *P < 0.0001. (B) NCAPG protein expression was evaluated by Western blotting in PC3, DU145, and C4‐2 cells 72 h after transfection with miR‐99a‐3p. (C) miR‐99a‐3p binding sites in the 3′‐UTR of NCAPG mRNA. Dual‐luciferase reporter assays in PC3 using vectors encoding a putative miR‐99a‐3p target site in the NCAPG 3′‐UTR (positions 462–468). Data were normalized by expression ratios of Renilla/firefly luciferase activities. *P < 0.0001.
To validate direct binding of miR‐99a‐3p in NCAPG mRNA, we performed luciferase reporter assays. The TargetScan database predicted that miR‐99a‐3p joined at position 462–468 in the 3′‐UTR of NCAPG. The luminescence intensity was remarkably reduced by cotransfection with miR‐99a‐3p and wild‐type vector of 3′‐UTR of NCAPG. In contrast, using the vector in which the target site of miR‐99a‐3p was deleted, the luminescence intensity did not change (Fig. 4C).
Expression of NCAPG in PCa clinical specimens
We evaluated the expression levels of NCAPG in PCa tissues (HSPC: n = 16, CRPC: n = 4), normal tissues (n = 17), and PCa cell lines (PC3, DU145, and C4‐2). NCAPG was markedly upregulated in CRPC tissues compared with that in normal tissues and HSPC tissues (P = 0.0002, P = 0.0018, respectively; Fig. 5A). Additionally, Spearman's rank test indicated that miR‐99a‐3p and NCAPG were expressed with negative correlation. (P = 0.0263, r = −0.370; Fig. 5B).
Figure 5.
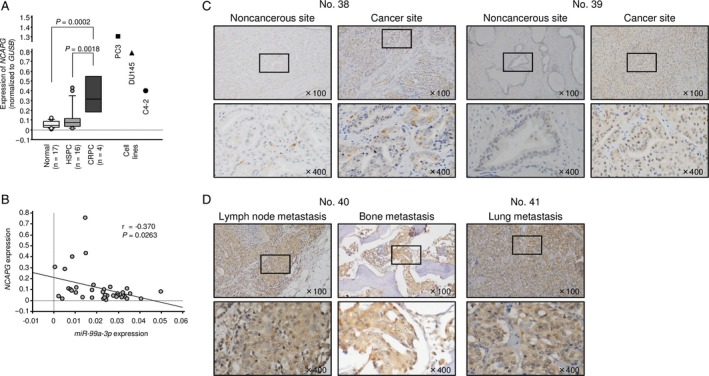
Expression of NCAPG in clinical PCa specimens. (A) Expression levels of NCAPG in PCa clinical specimens and cell lines. GUSB was used as an internal control. (B) The negative correlation between miR‐99a‐3p and NCAPG. (C) Immunochemical staining of NCAPG in HSPC specimens. (D) Immunochemical staining of NCAPG in mCRPC specimens.
Furthermore, to analyze NCAPG protein expression, immunohistochemistry was performed with PCa clinical specimens (Table 1). In CRPC specimens, NCAPG protein was strongly expressed in metastatic tissues from patients with CRPC, compared with non‐PCa or HSPC specimens (Fig. 5C and D).
Effects of silencing NCAPG in PCa cell lines
We examined the effects of NCAPG knockdown in PC3, DU145, and C4‐2 cells using two types of si‐NCAPG (si‐NCAPG‐1 and si‐NCAPG‐2). Two siRNAs effectively downregulated NCAPG mRNA and NCAPG protein expression in PC3, DU145, and C4‐2 cells (Fig. 6A and B). Additionally, functional assays indicated that cell proliferation, migration, and invasion were significantly inhibited by knockdown of NCAPG in comparison with mock‐ or si‐control‐transfected cells (Fig. 6C‐E, S5A and B). Even in LNCaP cells, cell proliferation assay was performed, and its ability was markedly suppressed by knockdown of NCAPG (data not shown).
Figure 6.
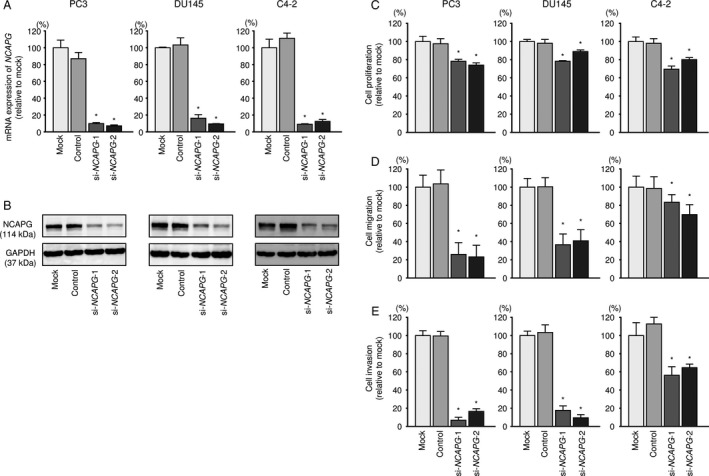
Effects of NCAPG silencing in PCa cell lines. (A) NCAPG mRNA expression was evaluated using qRT‐PCR analysis of PC3, DU145, and C4‐2 cells 48 h after transfection with si‐NCAPG‐1 or si‐NCAPG‐2. GAPDH was used as an internal control. *P < 0.0001. (B) NCAPG protein expression was evaluated by Western blot analysis of PC3, DU145, and C4‐2 cells 72 h after transfection with si‐NCAPG‐1 or si‐NCAPG‐2. GAPDH was used as a loading control. (C‐E) Cell proliferation, migration, and invasion assays following transfection with si‐NCAPG‐1 and si‐NCAPG‐2. *P < 0.0001.
Effects of cotransfection with NCAPG/miR‐99a‐3p in PC3 cells
We performed NCAPG rescue experiments by cotransfection with NCAPG and miR‐99a‐3p into PC3 cells. Western blot analysis of NCAPG protein expression is shown in Figure 7A and B. According to Western blotting, NCAPG protein levels were recovered by cotransfection with NCAPG and miR‐99a‐3p in PC3 cells. Moreover, the proliferation, migration, and invasion capacities of PC3 cells were recovered by cotransfection with NCAPG and miR‐99a‐3p compared with cells transfected with miR‐99a‐3p only (Fig. 7C–E, S6A and B). These results indicated that NCAPG affected the aggressiveness of PC3 cells.
Figure 7.
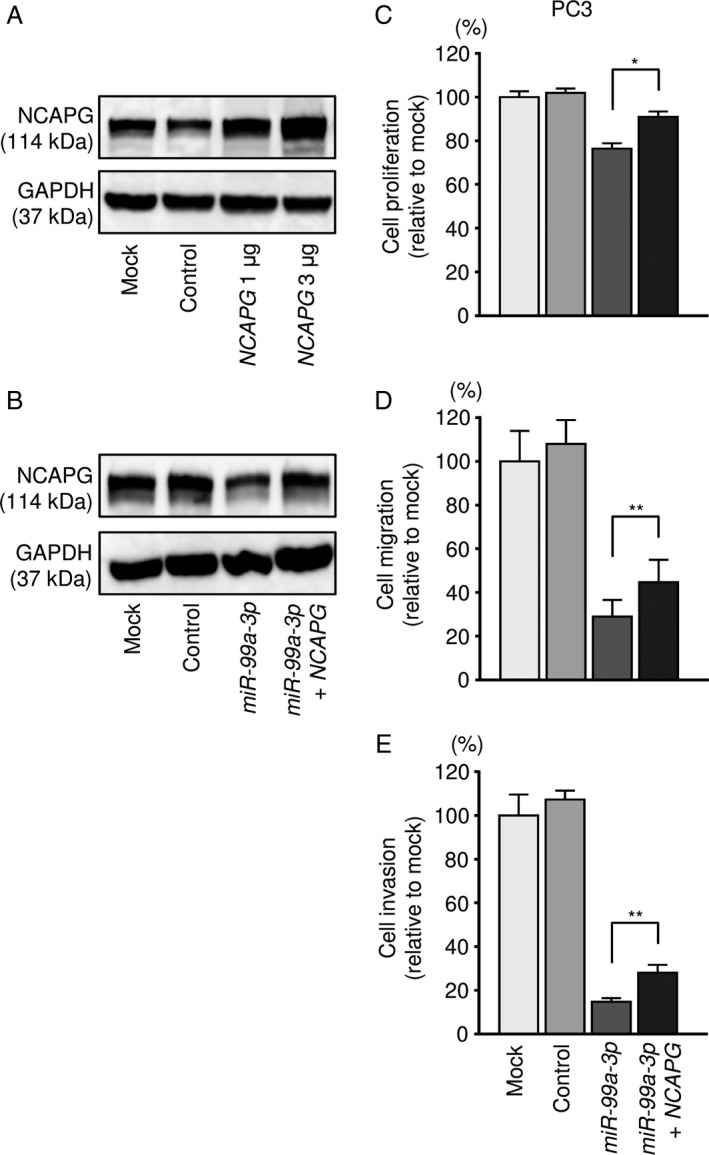
Effects of cotransfection with NCAPG/miR‐99a‐3p in PCa cell lines. (A) NCAPG protein expression was evaluated by Western blot analysis of PC3 cells 48 h after forward transfection with the NCAPG vector. GAPDH was used as a loading control. (B) NCAPG protein expression was evaluated by Western blot analysis of PC3 cells 72 h after reverse transfection with miR‐99a‐3p and 48 h after forward transfection with the NCAPG vector. (C) Cell proliferation was determined using XTT assays 72 h after reverse transfection with miR‐99a‐3p and 48 h after forward transfection with the NCAPG vector. *P < 0.0001. (D) Cell migration activity was assessed by wound‐healing assays 48 h after reverse transfection with miR‐99a‐3p and 24 h after forward transfection with the NCAPG vector. **P < 0.001. (E) Cell invasion activity was characterized by invasion assays 48 h after reverse transfection with miR‐99a‐3p and 24 h after forward transfection with the NCAPG vector. **P < 0.001.
Discussion
One of the main challenges in the treatment of CRPC is the control of aggressive and lethal metastatic PCa cells. We believe that identifying genes and pathways involved in metastasis and the acquisition of treatment resistance will lead to the development of new therapeutic strategies. Based on this background, we have identified several antitumor miRNAs, for example, miR‐1, miR‐133a, miR‐26a, miR‐26b, the miR‐29 family, miR‐205, miR‐218, miR‐221, miR‐222, miR‐223, and miR‐452, and showed that these miRNAs target oncogenes 24, 25, 28, 29, 30, 31, 32, 33, 34. Among these oncogenic genes, the extracellular matrix‐related genes laminin γ3 (LAMC3) and lysyl oxidase‐like 2 (LOXL2) were found to be overexpressed in naïve PCa clinical specimens and to enhance cancer cell migration and invasion in PCa cells 30, 31. Moreover, integrin α3 (ITGA3) and β1 (ITGB1), heterodimeric transmembrane receptors, were also overexpressed in naïve PCa clinical specimens, and integrin‐mediated oncogenic signaling enhanced cancer cell aggressiveness 25. These molecules are putative therapeutic targets for patients with naïve PCa and CRPC.
In general miRNA biogenesis, guide strand of miRNA is incorporated into RISC (RNA‐induced silencing complex) and acts as a fine‐tuner of expression control. In contrast, passenger strand of miRNA is disassembled and has no function 17, 18, 19. In miRNA biology, miRNA strand selection process is still obscure that which strand become the guide strand or the passenger strand from a miRNA duplex. Recent studies suggested that the thermodynamic character of the duplex seems to play an important role 35. An important feature of the miRNA guide strand is the U‐bias at the 5′end and excess purine, and the passenger strand has a C‐bias at the 5′ end and an excess of pyrimidine 35. The molecular dynamics of miRNA (guide strand and passenger strand) degradation and stabilization in normal and disease cells remain largely unknown.
Despite the previous theory that passenger strands of miRNA have no function, many studies have suggested that some passenger strands have actually functioning in the plant and human cells 36, 37, 38. Our recent studies showed that some passenger strands of miRNAs, for example, miR‐145‐3p, miR‐149‐3p, miR‐150‐3p, miR‐199a‐3p, and miR‐144‐5p, acted as antitumor miRNAs in several types of cancers 11, 12, 13, 14, 15. miR‐145‐5p (guide strand) is known to act as an antitumor miRNA in a variety of cancers through targeting several oncogenes 39, 40, 41. We showed that both strands of pre‐miR‐145, that is, miR‐145‐5p and miR‐145‐3p, were significantly downregulated in CRPC specimens compared with those in naïve PCa or non‐PCa specimens 15. Our data demonstrated that miR‐145‐3p (passenger strand) had stronger antitumor effects than miR‐145‐5p (guide strand) in PCa cells 15. We also confirmed the antitumor effects of miR‐145‐3p in bladder and lung and cancers 42, 43. More recently, we showed that both miR‐150‐5p (guide strand) and miR‐150‐3p (passenger strand) acted as antitumor miRNAs through targeting SPARC/osteonectin and cwcv and kazal‐like domains proteoglycan 1 (SPOCK1) in naïve PCa and CRPC cells 11. The involvement of passenger strand miRNAs in cellular processes regulation is a new conception in RNA research.
In this study, we focused on miR‐99a‐5p whose expression was significantly downregulated in our miRNA signature of metastatic CRPC and investigated the functional roles including passenger strand miR‐99a‐3p in PCa cells. As the results, we indicated that miR‐99a‐3p has potent antitumor effects in PCa cells. The expression levels of the two miRNAs, miR‐99a‐5p and miR‐99a‐3p, were obviously different in clinical specimens and cancer cell lines. We do not see any clear answer as to why this kind of difference will arise. This challenge is an important issue for miRNA research. In addition, a more detailed study on the concentration of miRNAs to be transfected into cancer cells and antitumor effects will be necessary.
The miR‐99a‐5p (guide strand) has been reported to have tumor‐suppressive roles in various types of cancers, including PCa 20, 21, 22, 23, 44. In nonsmall‐cell lung cancer, miR‐99a‐5p was reported to suppress cancer cell proliferation and metastasis by controlling the AKT1 signaling pathway and insulin‐like growth factor‐1 receptor, which could also serve as a diagnostic biomarker 23, 44. Additionally, several recent reports demonstrated the antitumor effects of miR‐99a‐5p on mammalian target of rapamycin (mTOR) regulation 20, 21, 22. For example, miR‐99a‐5p directly regulates the mTOR pathway to induce G1‐phase cell cycle arrest and suppress tumorigenicity in renal cell carcinoma 21. Additionally, in PCa, the miR‐99 family, including miR‐99a‐5p, directly targets the chromatin‐remodeling factors SMARCA5 and SMARCD1 and the growth regulatory kinase mTOR, suppresses the expression of PSA, and blocks PCa cell proliferation 45. Furthermore, inhibition of the miR‐99a/let‐7c/miR‐125b‐2 miRNA cluster promotes the induction of several androgen‐induced genes and stimulates the initiation and progression of PCa 46.
In contrast, the passenger strand miR‐99a‐3p has been reported as a diagnostic marker of the chemotherapy response in patients with advanced colorectal cancer 47; however, there are no reports examining the functional significance of miR‐99a‐3p in cancer cells. Our previous studies of miRNA signatures showed that miR‐99a‐3p was significantly downregulated in bladder cancer, renal cell carcinoma, and head and neck squamous cell carcinoma, suggesting miR‐99a‐3p has antitumor roles in these cancers 48, 49, 50. Moreover, TCGA database revealed that low expression of miR‐99a‐3p was significantly associated with poor prognosis in head and neck cancer and lung adenocarcinoma (Fig. S7). This is the first report demonstrating that miR‐99‐3p may function as an antitumor miRNA in naïve PCa and CRPC cells.
Unique nature of miRNA, single miRNA controls vast number of genes in normal and cancer cells. We performed gene expression analyses and in silico database search to identify miR‐99a‐3p regulated oncogenic genes in PCa cells. Interestingly, a large number of cohort analyses by TCGA database showed several targets were deeply involved PCa pathogenesis. These genes might be important tools for elucidating the molecular pathogenesis of PCa and CRPC.
In this study, by focusing on miR‐99a‐3p, which had not been well studied in previous reports, we found that NCAPG was directly regulated by miR‐99a‐3p in PCa cells. Overexpression of NCAPG was observed in CRPC clinical specimens, and its expression was found to be essential for PCa pathogenesis, as demonstrated by analysis of TCGA database. Interestingly, our previous study indicated that NCAPG was regulated by miR‐145‐3p in PCa cells 15. Thus, NCAPG is a candidate gene controlled by multiple antitumor miRNAs in CRPC, and its function in the pathogenesis of PCa may be important. However, the cancer‐promoting functions of this molecule are still not well known.
NCAPG is involved in mitotic chromosome condensation and is related to the cell cycle. Mitotic chromosome condensation is an essential cellular property of all proliferating cells and results in reconstitution of chromosomes into rod‐like mitotic chromosomes, ensuring separation of sister chromatids during cell division. In vertebrates, there are two types of condensin complexes, type I and II complexes, both of which contain nonstructural maintenance of chromosomes (non‐SMC) regulatory subunits. Defects in one of the subunits cause incomplete chromosome condensation 51, 52. NCAPG exists in the condensin I complex and is associated with proper segregation of sister chromatids in the condensation and fission of mitotic chromosomes 53. Previous studies showed that NCAPG was involved in the cell cycle and had cancer‐promoting functions in several types of cancers 54, 55. A recent study showed that knockdown of NCAPG induced apoptosis, reduced cancer cell survival, and suppressed the epithelial–mesenchymal transition (EMT) in cancer cells via upregulation of Bax, cleaved caspase‐3, and E‐cadherin and downregulation of cyclin A1, CDK2, Bcl‐2, N‐cadherin, and HOXB9 in hepatocellular carcinoma 55. Our present data showed that aberrant expression of NCAPG enhanced PCa cell aggressiveness. Thus, these data suggested that NCAPG had clinical significance in PCa pathogenesis and could have applications as a therapeutic target in CRPC.
In conclusion, both strands of pre‐miR‐99a, that is, miR‐99a‐5p and miR‐99a‐3p, were significantly reduced in naïve PCa and CRPC clinical specimens. The passenger strand, miR‐99a‐3p, had potent antitumor effects via targeting of the oncogene NCAPG in PCa cells. NCAPG was markedly elevated in CRPC and was involved in CRPC pathogenesis, suggesting that NCAPG could have applications as a therapeutic target in CRPC. The involvement of passenger strand miRNAs in cancer cells is novel concept of naïve PCa and CRPC pathogenesis.
Conflict of Interest
The authors declare no conflict of interests.
Supporting information
Figure S1. Schematic representation of the chromosomal location of human miR‐99a.
Figure S2. Expression levels of pri‐miR‐99a in PCa clinical specimens and cell lines.
Figure S3. Both strands of miR‐99a‐5p and miR‐99a‐3p incorporated into the RISC.
Figure S4. Phase micrographs of wound healing and invasion assays following transfection with miR‐99a‐5p/3p in PCa cell lines.
Figure S5. Phase micrographs of wound healing and invasion assays following transfection with si‐NCAPG in PCa cell lines.
Figure S6. Phase micrographs of wound healing and invasion assays following cotransfection with NCAPG/miR‐99a‐3p in PC3 cells.
Figure S7. Kaplan‐Meier survival curves based on miR‐99a‐3p expression in patients with Head and Neck squamous cell carcinoma and Lung adenocarcinoma.
Table S1. Product numbers of reagents.
Acknowledgments
This study was supported by KAKENHI grants 17K16778(B), 17K16777(B), 16K20125(B), 17K11160(C), 16H05462(B), and 15K10801(C).
Cancer Medicine 2018; 7(5):1988–2002
References
- 1. Siegel, R. L. , Miller K. D., and Jemal A.. 2017. Cancer statistics, 2017. CA Cancer J. Clin. 67:7–30. [DOI] [PubMed] [Google Scholar]
- 2. Crona, D. J. , and Whang Y. E.. 2017. Androgen receptor‐dependent and ‐independent mechanisms involved in prostate cancer therapy resistance. Cancers (Basel) 9:pii: E67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crawford, E. D. , Higano C. S., Shore N. D., Hussain M., and Petrylak D. P.. 2015. Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J. Urol. 194:1537–1547. [DOI] [PubMed] [Google Scholar]
- 4. Bartel, D. P. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. [DOI] [PubMed] [Google Scholar]
- 5. Filipowicz, W. , Bhattacharyya S. N., and Sonenberg N.. 2008. Mechanisms of post‐transcriptional regulation by microRNAs: are the answers in sight? Nat. Rev. Genet. 9:102–114. [DOI] [PubMed] [Google Scholar]
- 6. Friedman, R. C. , Farh K. K., Burge C. B., and Bartel D. P.. 2009. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 19:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nelson, K. M. , and Weiss G. J.. 2008. MicroRNAs and cancer: past, present, and potential future. Mol. Cancer Ther. 7:3655–3660. [DOI] [PubMed] [Google Scholar]
- 8. Iorio, M. V. , and Croce C. M.. 2009. MicroRNAs in cancer: small molecules with a huge impact. J. Clin. Oncol. 27:5848–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esquela‐Kerscher, A. , and Slack F. J.. 2006. Oncomirs ‐ microRNAs with a role in cancer. Nat. Rev. Cancer 6:259–269. [DOI] [PubMed] [Google Scholar]
- 10. Wiemer, E. A. 2007. The role of microRNAs in cancer: no small matter. Eur. J. Cancer 43:1529–1544. [DOI] [PubMed] [Google Scholar]
- 11. Okato, A. , Arai T., Kojima S., Koshizuka K., Osako Y., Idichi T., et al. 2017. Dual strands of pre‐miR150 (miR1505p and miR1503p) act as antitumor miRNAs targeting SPOCK1 in naive and castration‐resistant prostate cancer. Int. J. Oncol. 51:245–256. [DOI] [PubMed] [Google Scholar]
- 12. Okato, A. , Arai T., Yamada Y., Sugawara S., Koshizuka K., Fujimura L., et al. 2017. Dual strands of Pre‐miR‐149 inhibit cancer cell migration and invasion through targeting FOXM1 in renal cell carcinoma. Int. J. Mol. Sci. 18:pii: E1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koshizuka, K. , Hanazawa T., Kikkawa N., Arai T., Okato A., Kurozumi A., et al. 2017. Regulation of ITGA3 by the anti‐tumor miR‐199 family inhibits cancer cell migration and invasion in head and neck cancer. Cancer Sci. 108:1681–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matsushita, R. , Seki N., Chiyomaru T., Inoguchi S., Ishihara T., Goto Y., et al. 2015. Tumour‐suppressive microRNA‐144‐5p directly targets CCNE1/2 as potential prognostic markers in bladder cancer. Br. J. Cancer 113:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goto, Y. , Kurozumi A., Arai T., Nohata N., Kojima S., Okato A., et al. 2017. Impact of novel miR‐145‐3p regulatory networks on survival in patients with castration‐resistant prostate cancer. Br. J. Cancer 117:409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gregory, R. I. , Chendrimada T. P., Cooch N., and Shiekhattar R.. 2005. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123:631–640. [DOI] [PubMed] [Google Scholar]
- 17. Chendrimada, T. P. , Gregory R. I., Kumaraswamy E., Norman J., Cooch N., Nishikura K., et al. 2005. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutvagner, G. , and Zamore P. D.. 2002. A microRNA in a multiple‐turnover RNAi enzyme complex. Science 297:2056–2060. [DOI] [PubMed] [Google Scholar]
- 19. Matranga, C. , Tomari Y., Shin C., Bartel D. P., and Zamore P. D.. 2005. Passenger‐strand cleavage facilitates assembly of siRNA into Ago2‐containing RNAi enzyme complexes. Cell 123:607–620. [DOI] [PubMed] [Google Scholar]
- 20. Hu, Y. , Zhu Q., and Tang L.. 2014. MiR‐99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS ONE 9:e92099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cui, L. , Zhou H., Zhao H., Zhou Y., Xu R., Xu X., et al. 2012. MicroRNA‐99a induces G1‐phase cell cycle arrest and suppresses tumorigenicity in renal cell carcinoma. BMC Cancer 12:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao, J. , Chen F., Zhou Q., Pan W., Wang X., Xu J., et al. 2016. Aberrant expression of microRNA‐99a and its target gene mTOR associated with malignant progression and poor prognosis in patients with osteosarcoma. Onco. Targets Ther. 9:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen, C. , Zhao Z., Liu Y., and Mu D.. 2015. microRNA‐99a is downregulated and promotes proliferation, migration and invasion in non‐small cell lung cancer A549 and H1299 cells. Oncol. Lett. 9:1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goto, Y. , Kojima S., Kurozumi A., Kato M., Okato A., Matsushita R., et al. 2016. Regulation of E3 ubiquitin ligase‐1 (WWP1) by microRNA‐452 inhibits cancer cell migration and invasion in prostate cancer. Br. J. Cancer 114:1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kurozumi, A. , Goto Y., Matsushita R., Fukumoto I., Kato M., Nishikawa R., et al. 2016. Tumor‐suppressive microRNA‐223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 107:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arai, T. , Okato A., Kojima S., Idichi T., Koshizuka K., Kurozumi A., et al. 2017. Regulation of spindle and kinetochore‐associated protein 1 by antitumor miR‐10a‐5p in renal cell carcinoma. Cancer Sci. 108:2088–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao, J. , Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., et al. 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kojima, S. , Chiyomaru T., Kawakami K., Yoshino H., Enokida H., Nohata N., et al. 2012. Tumour suppressors miR‐1 and miR‐133a target the oncogenic function of purine nucleoside phosphorylase (PNP) in prostate cancer. Br. J. Cancer 106:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kato, M. , Goto Y., Matsushita R., Kurozumi A., Fukumoto I., Nishikawa R., et al. 2015. MicroRNA‐26a/b directly regulate La‐related protein 1 and inhibit cancer cell invasion in prostate cancer. Int. J. Oncol. 47:710–718. [DOI] [PubMed] [Google Scholar]
- 30. Kato, M. , Kurozumi A., Goto Y., Matsushita R., Okato A., Nishikawa R., et al. 2017. Regulation of metastasis‐promoting LOXL2 gene expression by antitumor microRNAs in prostate cancer. J. Hum. Genet. 62:123–132. [DOI] [PubMed] [Google Scholar]
- 31. Nishikawa, R. , Goto Y., Kojima S., Enokida H., Chiyomaru T., Kinoshita T., et al. 2014. Tumor‐suppressive microRNA‐29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int. J. Oncol. 45:401–410. [DOI] [PubMed] [Google Scholar]
- 32. Nishikawa, R. , Goto Y., Sakamoto S., Chiyomaru T., Enokida H., Kojima S., et al. 2014. Tumor‐suppressive microRNA‐218 inhibits cancer cell migration and invasion via targeting of LASP1 in prostate cancer. Cancer Sci. 105:802–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishikawa, R. , Goto Y., Kurozumi A., Matsushita R., Enokida H., Kojima S., et al. 2015. MicroRNA‐205 inhibits cancer cell migration and invasion via modulation of centromere protein F regulating pathways in prostate cancer. Int. J. Urol. 22:867–877. [DOI] [PubMed] [Google Scholar]
- 34. Goto, Y. , Kojima S., Nishikawa R., Kurozumi A., Kato M., Enokida H., et al. 2015. MicroRNA expression signature of castration‐resistant prostate cancer: the microRNA‐221/222 cluster functions as a tumour suppressor and disease progression marker. Br. J. Cancer 113:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Frank, F. , Sonenberg N., and Nagar B.. 2010. Structural basis for 5′‐nucleotide base‐specific recognition of guide RNA by human AGO2. Nature 465:818–822. [DOI] [PubMed] [Google Scholar]
- 36. Liu, W. W. , Meng J., Cui J., and Luan Y. S.. 2017. Characterization and Function of MicroRNA(*)s in Plants. Front. Plant Sci. 8:2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCall, M. N. , Kim M. S., Adil M., Patil A. H., Lu Y., Mitchell C. J., et al. 2017. Toward the human cellular microRNAome. Genome Res. 27:1769–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marzi, M. J. , Ghini F., Cerruti B., de Pretis S., Bonetti P., Giacomelli C., et al. 2016. Degradation dynamics of microRNAs revealed by a novel pulse‐chase approach. Genome Res. 26:554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Feng, Y. , Zhu J., Ou C., Deng Z., Chen M., Huang W., et al. 2014. MicroRNA‐145 inhibits tumour growth and metastasis in colorectal cancer by targeting fascin‐1. Br. J. Cancer 110:2300–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Avgeris, M. , Stravodimos K., Fragoulis E. G., and Scorilas A.. 2013. The loss of the tumour‐suppressor miR‐145 results in the shorter disease‐free survival of prostate cancer patients. Br. J. Cancer 108:2573–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cui, X. B. , Li S., Li T. T., Peng H., Jin T. T., Zhang S. M., et al. 2016. Targeting oncogenic PLCE1 by miR‐145 impairs tumor proliferation and metastasis of esophageal squamous cell carcinoma. Oncotarget 7:1777–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsushita, R. , Yoshino H., Enokida H., Goto Y., Miyamoto K., Yonemori M., et al. 2016. Regulation of UHRF1 by dual‐strand tumor‐suppressor microRNA‐145 (miR‐145‐5p and miR‐145‐3p): Inhibition of bladder cancer cell aggressiveness. Oncotarget 7:28460–28487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mataki, H. , Seki N., Mizuno K., Nohata N., Kamikawaji K., Kumamoto T., et al. 2016. Dual‐strand tumor‐suppressor microRNA‐145 (miR‐145‐5p and miR‐145‐3p) coordinately targeted MTDH in lung squamous cell carcinoma. Oncotarget 7:72084–72098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu, S. H. , Zhang C. L., Dong F. S., and Zhang Y. M.. 2015. miR‐99a suppresses the metastasis of human non‐small cell lung cancer cells by targeting AKT1 signaling pathway. J. Cell. Biochem. 116:268–276. [DOI] [PubMed] [Google Scholar]
- 45. Sun, D. , Lee Y. S., Malhotra A., Kim H. K., Matecic M., Evans C., et al. 2011. miR‐99 family of MicroRNAs suppresses the expression of prostate‐specific antigen and prostate cancer cell proliferation. Cancer Res. 71:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun, D. , Layer R., Mueller A. C., Cichewicz M. A., Negishi M., Paschal B. M., et al. 2014. Regulation of several androgen‐induced genes through the repression of the miR‐99a/let‐7c/miR‐125b‐2 miRNA cluster in prostate cancer cells. Oncogene 33:1448–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Molina‐Pinelo, S. , Carnero A., Rivera F., Estevez‐Garcia P., Bozada J. M., Limon M. L., et al. 2014. MiR‐107 and miR‐99a‐3p predict chemotherapy response in patients with advanced colorectal cancer. BMC Cancer 14:656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Itesako, T. , Seki N., Yoshino H., Chiyomaru T., T. Yamasaki , Hidaka H., et al. 2014. The microRNA expression signature of bladder cancer by deep sequencing: the functional significance of the miR‐195/497 cluster. PLoS ONE 9:e84311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goto, Y. , Kurozumi A., Nohata N., Kojima S., Matsushita R., Yoshino H., et al. 2016. The microRNA signature of patients with sunitinib failure: regulation of UHRF1 pathways by microRNA‐101 in renal cell carcinoma. Oncotarget 7:59070–59086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koshizuka, K. , Nohata N., Hanazawa T., Kikkawa N., Arai T., Okato A., et al. 2017. Deep sequencing‐based microRNA expression signatures in head and neck squamous cell carcinoma: dual strands of pre‐miR‐150 as antitumor miRNAs. Oncotarget 8:30288–30304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hirano, T. , Kobayashi R., and Hirano M.. 1997. Condensins, chromosome condensation protein complexes containing XCAP‐C, XCAP‐E and a Xenopus homolog of the Drosophila Barren protein. Cell 89:511–521. [DOI] [PubMed] [Google Scholar]
- 52. Thadani, R. , Uhlmann F., and Heeger S.. 2012. Condensin, chromatin crossbarring and chromosome condensation. Curr. Biol. 22:R1012–R1021. [DOI] [PubMed] [Google Scholar]
- 53. Herzog, S. , Nagarkar Jaiswal S., Urban E., Riemer A., Fischer S., and Heidmann S. K.. 2013. Functional dissection of the Drosophila melanogaster condensin subunit Cap‐G reveals its exclusive association with condensin I. PLoS Genet. 9:e1003463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liang, M. L. , Hsieh T. H., Ng K. H., Tsai Y. N., Tsai C. F., Chao M. E., et al. 2016. Downregulation of miR‐137 and miR‐6500‐3p promotes cell proliferation in pediatric high‐grade gliomas. Oncotarget 7:19723–19737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu, W. , Liang B., Liu H., Huang Y., Yin X., Zhou F., et al. 2017. Overexpression of nonSMC condensin I complex subunit G serves as a promising prognostic marker and therapeutic target for hepatocellular carcinoma. Int. J. Mol. Med. 40:731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic representation of the chromosomal location of human miR‐99a.
Figure S2. Expression levels of pri‐miR‐99a in PCa clinical specimens and cell lines.
Figure S3. Both strands of miR‐99a‐5p and miR‐99a‐3p incorporated into the RISC.
Figure S4. Phase micrographs of wound healing and invasion assays following transfection with miR‐99a‐5p/3p in PCa cell lines.
Figure S5. Phase micrographs of wound healing and invasion assays following transfection with si‐NCAPG in PCa cell lines.
Figure S6. Phase micrographs of wound healing and invasion assays following cotransfection with NCAPG/miR‐99a‐3p in PC3 cells.
Figure S7. Kaplan‐Meier survival curves based on miR‐99a‐3p expression in patients with Head and Neck squamous cell carcinoma and Lung adenocarcinoma.
Table S1. Product numbers of reagents.


