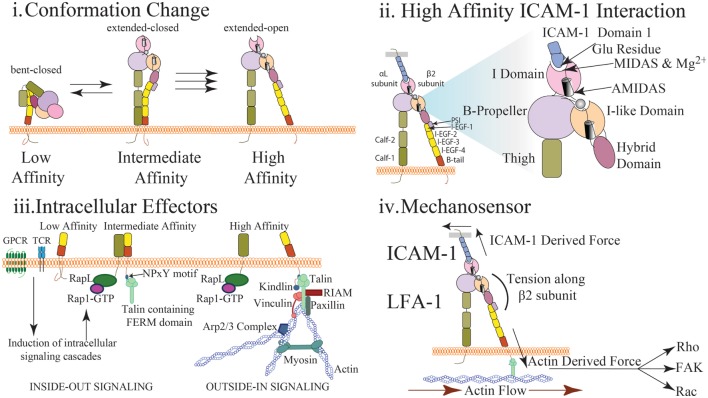
Figure 1.
Multidimensional regulation of LFA-1 affinity (i) LFA-1 affinity regulation is mediated via conformational changes to LFA-1 structure. In the low affinity state, the bent conformation causes the ligand binding αI domain to be inaccessible to interact with ICAM-1. In the intermediate affinity state, the extracellular leg domains are straightened allowing for low affinity interactions between LFA-1 and ICAM-1. Importantly, the intracellular domains of LFA-1 are not separated and the metal ion-dependent adhesion site (MIDAS) binding site closed. In the high affinity state, disruption of the salt bridge between the α and β cytosolic tails results in conformational shift along the β subunit and αI domain resulting in high affinity LFA-1 via the opening of the ligand-binding site. (ii) The αI domain contains the MIDAS within which resides Mg2+ coordinating the binding pocket. This site interacts with the glutamic acid-34 in Domain 1 of ICAM-1 to facilitate binding. This induces a shift in the α7 helix to cause the hybrid domain to swing out further stabilizing LFA-1 structure. Additional sites surrounding the MIDAS such as AMIDAS and ligand-induced metal-binding site assist with coordination of the binding pocket and stabilization of high affinity LFA-1. (iii) Upon T cell receptor or chemokine activation, RAP1-GTP recruits a number of factors including RAPL that interact with the α subunit of LFA-1 to induce integrin activation (inside-out signaling). Similarly, talin cleavage allows the FERM domain to interact with the NPxY motif of the cytosolic tail on the β subunit. This interaction causes a dissociation of the salt bridge inducing cytosolic tail separation. Kindlin also contains a FERM domain and interacts with the β subunit to further stabilize high affinity LFA-1. Molecules such as RIAM, talin, paxillin, and vinculin may interact with the cytosolic tails to recruit additional effector molecules and promote a scaffold to interact with actin and reinforce LFA-1 activity (outside-in signaling). Arp2/3 will promote continued actin filament growth while MyH9 functions to provide stress on actin fibers to induce LFA-1 dissociation from ligand. (iv) Interaction of LFA-1 with ICAM-1 and β-actin allows for force driven responses along the β subunit. Transmission of force (arrows) along the β-subunit has been measured in pN scale with actin flow functioning to direct the orientation and location of LFA-1 both at the immunological synapse and during cell migration. Stabilization of the integrin in the high affinity conformation via force generation requires adhesion to both the cytoskeleton and ICAM-1. The stiffness of the substrate may also alter the level of force generated thus altering the signaling response. Downstream signal is induced via outside in signaling generated through the stabilization of high affinity LFA-1. Phosphorylation of focal adhesion kinase through force generation may play a role in mediating cell adhesion and proliferation. Rho signaling, and thus actin polymerization, may also be altered through changes in force generation resulting in changes in actin dynamics and cell migration. Induction of Rac and CDC42 may also be altered through force generation resulting in changes to cell proliferation and survival.