Abstract
To define the optimal systolic phase for dual-source computed tomography angiography using an absolute reconstruction delay time after the R–R interval based on the coronary artery motion, we analyzed images reconstructed between 200 and 420 miliseconds (ms) after the R wave at 20 ms increments in 21 patients. Based on the American Heart Association coronary segmentation guidelines, the origin of six coronary artery landmarks (RCA, AM1, PDA, LM, OM1, and D2) were selected to calculate the coronary artery motion velocity. The velocity of the given landmark was defined as the quotient of the route and the length of the time interval. The x, y and z-coordinates of the selected landmark were recorded, and were used for the calculation of the 3D route of coronary artery motion by using a specific equation. Differences in velocities were assessed by analysis of variance for repeated measures; Bonferroni post hoc tests were used for multiple pair wise comparisons. 1488 landmarks were measured (6 locations at 12 systolic time points) in 21 patients and were analyzed. The mean values of the minimum velocities were calculated separately for each heart rate group (i.e. <65; 65–80; and >80 bpm). The mean lowest coronary artery velocities in each segment occurred in the middle period of each time interval of the acquired systolic phase i.e. 280–340 ms. No differences were found in the minimal coronary artery velocities between the three HR groups, with the exception of the AM1 branch (p = 0.00495) between <65 and >80 bpm (p = 0.03), and at HRs of 65–80 versus >80 bpm (p = 0.006). During an absolute delay of 200–420 ms after the R-wave, the ideal reconstruction interval varies significantly among coronary artery segments. Decreased velocities occur between 280 to 340 ms. Therefore a narrow range of systolic intervals, rather than a single phase, should be acquired.
Keywords: Coronary CT angiography, Dual-source CT, Systolic phase targets, Absolute delay time
Introduction
Coronary computed tomography angiography (CTA) is an established noninvasive study to evaluate coronary artery disease and atherosclerosis. CTA is a technically demanding procedure, and motion artifacts present the chief challenge unique to ECG-gated coronary CTA [1, 2]. While the temporal resolution of CT has dramatically improved in the four decades since its debut, acquisition times are still significantly limited as compared to the reference standard, high-frame-rate cine fluoroscopy. Diagnostic image quality can be achieved by synchronizing the acquisition window to the phase of the cardiac cycle with minimal coronary arterial motion. Thus cardiac coronary CTA image acquisitions are typically performed in the most quiescent period of the cardiac cycle, which at low and stable heart rates (HR) is during mid-diastole (during diastasis) [3–5]. While the duration of this period is relatively lengthy and predictable in patients with low and stable heart rates, at higher heart rates the length of diastasis significantly shortens and eventually disappears [6]. Hence coronary CTA is customarily performed in selected patients with favorable heart rates and rhythms, and premedication is often required to induce bradycardia, usually via blockade of beta-adrenergic receptors. β-blockers are safe and efficacious in most patients, but contraindications such as reactive airway disease and hypotension are occasionally a serious challenge [7]. There are some patients in whom the receptor’s response to β-blocker cannot be predicted and they remain in higher range of heart rate in spite of high dose administration. Additionally, irregularity of the cardiac cycle cannot always be rectified by medication.
Alternatively, the end-systolic phase is a second relatively tranquil phase where data acquisition is suitable. This period is also less sensitive to R–R variability and arrhythmia as compared to diastole [8]. For example, the length of systole shortens by 5.6 % as heart rates increase from 80 to 90 beats per minute (bpm), while at the same time the length of diastole decreases by 16.4 % [9]. Thorough prior investigations have established that image reconstruction using diastolic data is favorable at heart rates under 65 bpm [4, 10, 11]. While at increased heart rates, the end-systolic and early-diastolic reconstructions are more favorable [10, 12, 13]. Further, the length of systole is a relatively fixed phenomenon, and end systole can be targeted by using absolute delay times, as opposed to defining the timing of systole as a fraction of the cardiac cycle. Lee et al. [14] studied the image quality during the end-systolic temporal window in patients with various heart rates, finding image quality and radiation dose advantages across a wide range of heart rates. The ideal phase targets within systole at various heart rates have not yet been defined as opposed to diastolic targets, which have been carefully evaluated in several studies of patients with low, steady heart rates, using several different CT systems.
Coronary artery motion and deformation during the cardiac cycle leads to significant motion artifacts when velocity exceeds the temporal resolution of the image reconstruction [15]. Therefore, the aim of our study was to identify whether optimal systolic phase targets could be identified using an absolute reconstruction delay time within the R–R interval, by evaluating coronary arterial velocities at various heart rates.
Materials and methods
This study was approved by the human research committee of the institutional review board (IRB), and compliance with the Health Insurance Portability and Accountability Act guidelines was maintained. A waiver of consent was obtained from our local IRB for this retrospective study. The authors maintained full control over the study design and data.
Patient population
This retrospective cohort study consisted of 21 selected patients (14 men, 7 women; mean age 53.6 years ± 13.1; age range, 29–78 years) who were referred for clinically indicated native coronary CTA between November 2012 and May 2013. Patient selection was based on a clinical decision to target a systolic image reconstruction time interval (from at least 200–420 ms). None of the selected patients had coronary anomalies, nor had undergone coronary artery bypass grafting or prior electrophysiological interventions (such as ablation procedures, pacemaker implantation, or defibrillator implantation).
Patients were divided into three groups based on mean heart rate: <65; 65–80; or >80 bpm. The mean HR was 71 bpm (range 52–96 bpm).
CT data acquisition and image analysis
All examinations were performed on a second-generation dual-source 128-slice CT scanner (SOMATOM Definition Flash, Siemens Medical Systems, Forchheim, Germany, software update VA40) with the following acquisition parameters: 128 slices at 0.6 mm thickness (using a z-axis flying focal spot) and gantry rotation time of 280 ms (and resultant temporal resolution of 75 ms).
Prospectively ECG-triggered axial-sequential acquisition (Sequential Scanning; Siemens) with an advanced arrhythmia rejection algorithm mode (Adaptive Cardio Sequence, “Adaptive Cardio Sequential Flex mode”, Siemens) was used in 18 scans, which was enabled to reject and re-acquire data at table positions scanned during heartbeats falling outside of a pre-specified cardiac cycle length. Systolic acquisition was performed as previously described using an absolute delay of 200–450 ms after the R-wave with peak (100 % of prescribed reference) tube current from 300 to 400 ms and a baseline plateau (20 % of the reference tube current) in the other prescribed phases [16].
Three patients underwent retrospectively ECG-gated CTA with peak targets in the same phases of systole with aggressive (“MinDose”, Siemens) tube modulation outside of the 200–450 ms window.
In patients without contraindications, 0.6 mg of sublingual nitroglycerine was administered approximately 5 min prior to scanning. Importantly, none of the patients received β-blockers during the examination, which was a decision at the discretion of the supervising CT physician as per standard site practice. However, nine (43 %) patients’ home baseline regimen included oral β-receptor blockers.
Arterial phase contrast was timed using the test bolus method using 20 ml iodinated contrast media (Iopamidol 370 g/cm3, Bracco Diagnostics Princeton, NJ USA) injected at a rate of 4–7 ml/sec (based on body-mass index and IV access as per clinical routine) via an antecubital vein using a power injector, with diagnostic injections based upon scan time length. All injections were followed by a 40 ml of normal saline flush at a matching flow rate.
Tube potential (kV) and tube current (mAs) were calculated by an automatic tube potential selection algorithm based on AP scout image characteristics (CAREDose 4D and CAREkV, Siemens) [17]. All scans were supervised by a cardiovascular imaging specialist (at least one board-certified or eligible radiologist or cardiologist with advanced training in cardiac CT).
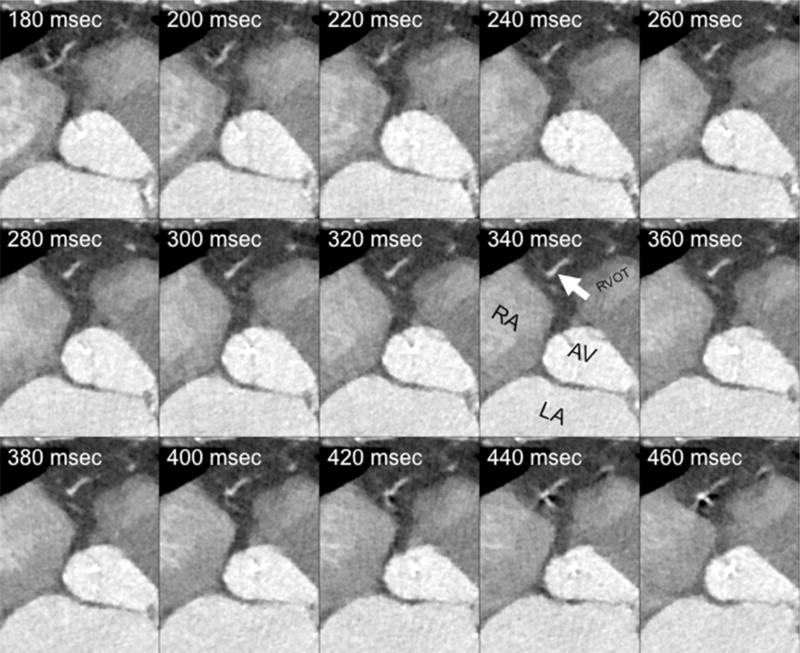
Raw datasets were reconstructed in 20 ms increments between 200 to 440 ms after the R peak using 1 mm thick slices and archived to the picture archival and communication system (PACS) (Fig. 1). Images were retrieved and displayed on a 3-D workstation (Osirix v. 3.7.1 32-bit, Pixmeo, Geneva, Switzerland). Image review included axial source images, orthogonal and oblique multiplanar reformatted (MPR) images, and thin slab maximum intensity projection (MIP) images, while advancing manually through the various acquired phases in 4-dimensional (cine) mode.
Fig. 1.
Raw data sets reconstructions in 20 ms increments after R peak with 1 mm thick slices. Mulitple axial reconstructions of the right heart during systole (absolute delays after the R-wave from 180 ms through 460 ms in 20 ms increments) demonstrate the optimal phase time of 340 ms at the level of an acute marginal branch (white arrow). The right atrium (RA), left atrium (LA), aortic valve (AV), and right ventricular outflow tract (RVOT) are marked
Coronary artery velocity mapping
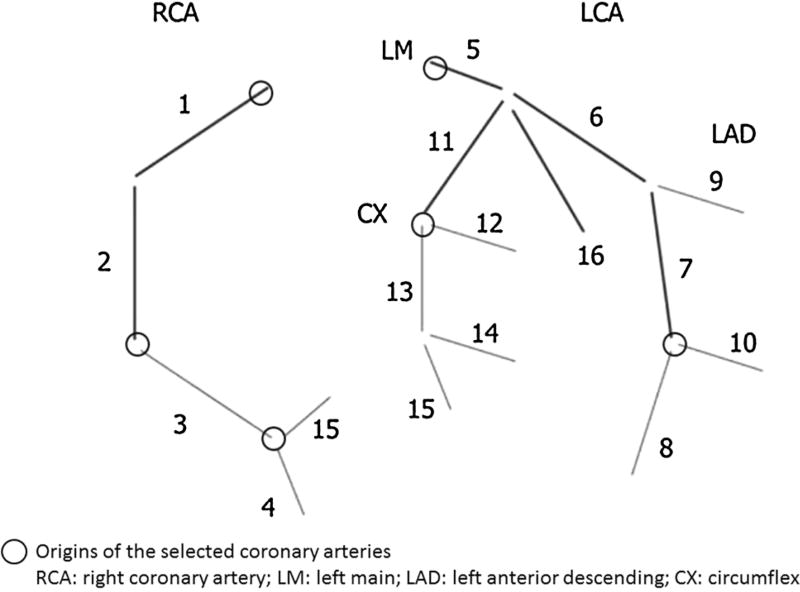
Based on the American Heart Association (AHA) coronary segmentation guidelines, six coronary artery landmarks (RCA-ostial right coronary artery, AM1-first acute marginal branch, PDA-posterior descending artery, LM-left main coronary artery, OM1-first obtuse marginal branch, and D2-second diagonal branch) were identified in each patient, in 20 ms increments, by two experienced physicians (HV and CC) [18] (Fig. 3).
Fig. 3.
Landmark position for manual velocity mapping based on the AHA coronary segmentation guidelines
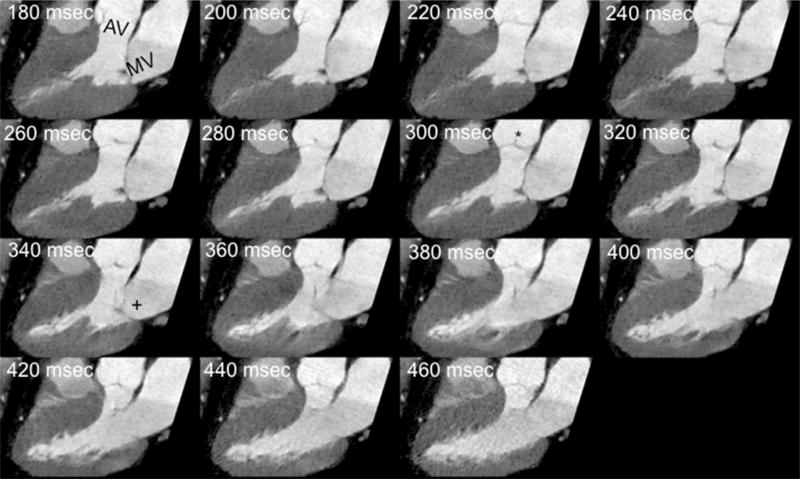
The end systolic phase and the end of isovolumic relaxation phases were identified with the aortic valve closure and initiation of mitral valve opening on the 3 chamber cine view as demonstrated in Fig. 2. The landmark’s position was traced manually by placing the cursor in the exact center of each landmark in every phase. The x, y and z-coordinates of the selected landmark were then recorded, and were used for the calculation of the 3D route of coronary artery motion by using a previously established methods [19]: the velocity of the given landmark was defined as the quotient of the route and the length of the time interval, which was 20 ms.
Fig. 2.
Defining end systolic phase and the end of isovolumic relaxation phases following the aortic valve closure and initiation of mitral valve opening on the 3 chamber cine view. Three-chamber cine view reconstructed throughout the acquired systolic intervals demonstrate the aortic (AV) and mitral (MV) valves. Aortic valve closure can be identified (asterisk, 300 ms reconstruction) and the initiation of mitral valve opening (plus, 340 ms reconstruction), which denote the end of the systolic reduced ejection phase, and the end of the isovolumetric relaxation phases, respectively
To determine the optimal velocities in each coronary artery segment, the mean values of the minimum velocities were calculated separately for each heart rate group (i.e. <65; 65–80; and >80 bpm).
The ideal reconstruction times in each heart rate group were defined as the R–R interval with the lowest minimum mean velocity in a given segment.
A preliminary analysis of 10 patients revealed that the mean lowest coronary artery velocities in each segment occurred in the middle of the selected time interval. Therefore we divided this interval into three sections from: 200 to 260 ms (early), 280–340 ms (mid) and 360–420 ms (late), to observe if any significant difference existed between the three sections.
Statistical analysis
Normality of variables was assessed using the Shapiro–Wilk test. Correlations were calculated with Pearson’s correlation test or Spearmans rho test as appropriate. To compare multiple variables one-way ANOVA, followed by Bonferroni post hoc test, or Kruskal–Wallis ANOVA followed by Mann–Whitney-U test was used depending on normality. Within subjects, measurements were compared using repeated measures ANOVA. Data are presented as mean ± standard deviation (SD), or median (interquartile range) for non-normally distributed data. A p value under 0.05 was used to define statistical significance.
Results
In total 1488 landmarks were available (24 of the various landmark data points were deemed non-evaluable or missing), in 21 patients at 6 locations throughout the coronary artery tree, at 12 time-points throughout the systolic phase reconstructions.
No significant correlation was found between coronary artery velocities and HR. (RCA: r = −0.75, p = 0.75; AM1: r = −0.48, p = 0.029; PDA: r = −0.31, p = 0.17; LM: r = −0.14; p = 0.54; OM1: r = −0.17, p = 0.45; D2: r = −0.16, p = 0.48).
Table 1 lists the minimal velocities in each segment for each HR group.
Table 1.
Optimal coronary artery velocities for each heart rate group
| <65 | 65–80 | >80 | All | p value | |
|---|---|---|---|---|---|
| RCA | 14.2 | 12.4 | 12.6 | 13.1 | 0.88000 |
| AM1 | 19.5 | 21.9 | 9.4 | 16.9 | 0.00495 |
| PDA | 22.1 | 15.8 | 15.0 | 17.7 | 0.41300 |
| LM | 12.9 | 11.9 | 11.5 | 12.1 | 0.94000 |
| OM1 | 14.0 | 13.8 | 11.8 | 13.2 | 0.77400 |
| D2 | 14.1 | 12.6 | 12.7 | 13.1 | 0.88400 |
RCA ostial right coronary artery, AM1 first acute marginal branch, PDA posterior descending artery, LM left main coronary artery, OM1 first obtuse marginal branch, D2 second diagonal branch. All velocities listed represent mm/s
No differences were found in the minimal coronary artery velocities between the three HR groups, with the exception of the AM1 branch (p = 0.00495) between <65 and >80 bpm (p = 0.03), and at HRs of 65–80 versus >80 bpm (p = 0.006).
Table 2 shows the optimal systolic phase reconstruction times of the evaluated coronary artery segments in ms.
Table 2.
Optimal systolic phase reconstruction time (ms)
| <65 | 65–80 | >80 | All | p value | |
|---|---|---|---|---|---|
| RCA | 326 | 357 | 269 | 317 | 0.0192 |
| AM | 343 | 311 | 334 | 329 | 0.451 |
| PDA | 326 | 274 | 277 | 292 | 0.177 |
| LM | 300 | 263 | 257 | 273 | 0.249 |
| OM | 311 | 343 | 289 | 314 | 0.294 |
| S2 | 277 | 306 | 303 | 295 | 0.542 |
RCA ostial right coronary artery, AM1 first acute marginal branch, PDA posterior descending artery, LM left main coronary artery, OM1 first obtuse marginal branch, D2 second diagonal branch. All times listed represent absolute delay after the R-wave (ms)
Significant differences in optimal reconstruction time points were detected only in the RCA (p = 0.019) between heart rates of 65–80 versus >80 bpm (p = 0.019), with a lower velocity at HR >80 versus 65–80 (269 vs. 357 ms).
Tables 1 and 2 indicate that coronary artery minimal velocities and optimal time-points are independent of HR. However, note that in two segments (AM1 and RCA) significant differences were demonstrated.
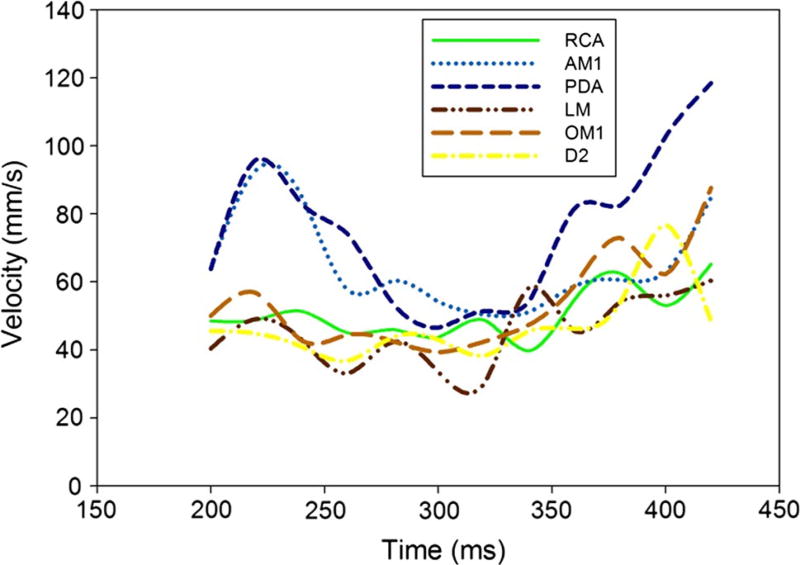
A preliminary analysis of ten patients revealed that the mean lowest coronary artery velocities in each segment occurred in the middle period of each time interval of the acquired systolic phase. Therefore we divided this interval into three time-periods from: 200 to 260 ms (early), 280–340 ms (mid) and 360–420 ms (late), to evaluate potential differences. The analysis of all 21 patients’ mean velocities in each of the three time periods confirmed this observation. In the mid period, (280–340 ms) in each coronary segment, the mean velocity values were significantly lower versus the early and late phases of systole. (Table 3, Fig. 4) In the PDA, LM, OM1 and D2, a significant difference was also found between the early and late time periods.
Table 3.
Differences between the systolic phase targets
| Coronary artery segment | Time interval (ms) | Mean velocity (mm/s) | p value | p value between time intervals |
|---|---|---|---|---|
| RCA | Early (200–260 ms) | 48.4 | Mid versus late p = 0.0047 | |
| Mid (280–340 ms) | 44.6 | |||
| late (360–420 ms) | 58.9 | |||
| p = 0.0048 | ||||
| AM1 | Early (200–260 ms) | 75.0 | Mid versus early p = 0.0039 | |
| Mid (280–340 ms) | 54.0 | |||
| Late (360–420 ms) | 66.4 | |||
| p = 0.0054 | ||||
| PDA | Early (200–260 ms) | 79.2 | Mid versus early p = 0.0155 | |
| Mid (280–340 ms) | 51.5 | |||
| Late (360–420 ms) | 95.6 | Mid versus late p = 0.000042 | ||
| p = 0.000061 | ||||
| LM | Early (200–260 ms) | 41.4 | Early versus late p = 0.00305 | |
| Mid (280–340 ms) | 41.1 | Mid versus late p = 0.00228 | ||
| late (360–420 ms) | 53.8 | |||
| p = 0.000687 | ||||
| OM1 | Early (200–260 ms) | 48.4 | Early versus late p = 0.0015 | |
| Mid (280–340 ms) | 42.9 | Mid versus late p = 0.000044 | ||
| Late (360–420 ms) | 70.2 | |||
| p = 0.00003 | ||||
| D2 | Early (200–260 ms) | 42.0 | Early versus late p = 0.0065 | |
| Mid (280–340 ms) | 42.6 | Mid versus late p = 0.0099 | ||
| Late (360–420 ms) | 56.0 | |||
| p = 0.00263 |
Fig. 4.
Mean coronary artery velocities in each of the three time periods. The lowest coronary velocity was detected in the mid period of the reconstructed interval (280–340 ms). The mean velocities in this period are significantly lower than in the early and/or late periods
Discussion
We found that coronary artery velocity during late systole and early diastole is independent of heart rate and in the mid period (260–340 ms absolute delay after the R-wave), the mean velocities were significantly lower, while also noting that a single specific reconstruction interval was not universally applicable. The relatively fixed length of the systole versus diastole is a well-understood phenomenon. At higher heart rates, the diastasis period shortens, and above heart rates of 96 bpm it eventually disappears [6, 20, 21]. Thus, when necessary, cardiac CT phase reconstructions at end-systole are often considered. Several previous studies have investigated image acquisitions performed during this period [10, 13, 22–24]. All of these investigations reported comparable or better image quality, with less variability and fewer motion artifacts than diastole at high and variable heart rates. Moreover, Okada et al. [25] observed that systolic reconstructions in addition to diastolic reconstructions were needed to obtain high quality coronary CT images in patients with high heart rate variability having high blood pressure and who are on medication for diabetes mellitus.
Based upon these prior studies, most of the clinically performed acquisitions included in our study were performed during systole by using prospectively-ECG triggered acquisition method. A prospectively ECG-triggered acquisition (targeted in any phase) in conjunction with automated tube potential current selection algorithm has the advantage of a consistent radiation exposure, due to the very minimal overlap of “slab” acquisitions in axial-sequential modes tailored to patient’s specific body size [14, 17, 26].
The selected absolute delay interval between 200 ms to 420 ms corresponds to the time-interval of the ventricular systole and extends between the peak of the R-wave and the T-wave or the descending T-wave of the electrocardiogram. Physiologically, ventricular systole is divided into two periods: the isovolumic contraction phase and the ejection phase. The ejection phase consists of an early phase when the maximum ejection occurs and a latter phase with reduced or absent ejection [11]. The reduced phase is immediately followed by the proto-diastole and the isovolumic relaxation. Previous physiological investigations revealed an inert systolic phase with a constant low motion at the end of systole and early diastole, thus providing a basis for late-systolic/early diastolic cardiac CT acquisitions [27].
Fabian et al. [28] sought to assess the durations of the left ventricular systolic phases, including the isovolumic contraction time (ICT), the pre-ejection period (PEP) and the left ventricular ejection time (LVET). According to their measurements, the mean ICT was 70 ± 9.5 ms with a range of 51–90 ms. The mean PEP was 100 ± 13 ms with a range of 78–130 ms and the mean LVET was 281 ± 21 ms, ranging from 230 to 334 ms.
In our study we found that the optimal time points with lowest coronary motion ranged from an average phase start time of 273 ms in the origin of the LM to 329 ms in the AM branch (Table 2), using images with a temporal window of 75 ms, noting that these artificial “mean” values may not apply specifically to a given patient. These findings are congruent with our findings that the mean coronary artery motion velocities were significantly lower in the mid period of the selected temporal window, between 280 and 340 ms (Fig. 3).
Thus we found that the lowest coronary motion occurs during the LVET, in its second half during reduced ejection through the following proto-diastole, and confirms the previous works reporting the existence of an inert constant low motion end-systolic early diastolic temporal window.
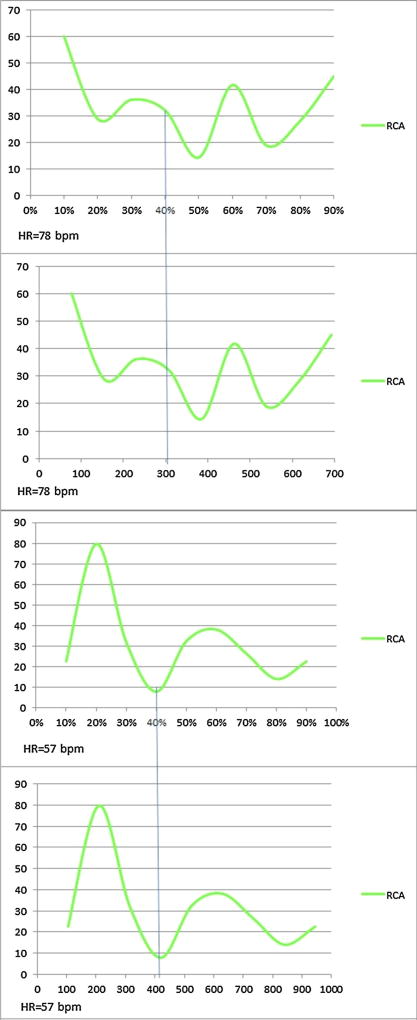
In cardiac CT, several image reconstruction algorithms related to ECG signal can be utilized to obtain diagnostic image quality [29]. Typically images are reconstructed during the least motion or between the T and P waves. The most frequently used approach is the relative delay method, in which the reconstruction starts after a certain delay from the prior wave which is ascribed as a certain percentage of the R–R interval. Another method is the absolute delay method in which reconstruction starts at a fixed time delay before or after the R wave and ascribed as a specific time delay in milliseconds [30]. Of note, comparisons of the two image reconstruction techniques have been infrequently investigated. Boehm et al. [31] directly compared the image quality and artifacts of the aortic and mitral valves when performing ECG-synchronization using relative and absolute delay reconstructions. Their results indicate that the absolute delay image reconstruction provides superior image quality with less motion artifacts. These differences are due to heart rate variability, as in patients with higher heart rates the diastole shortens which leads to the non-proportional decrease of the RR interval [11]. For example, in a patient with heart rate of 78 bpm (average R–R cycle length of 769 ms), a 40 % relative R–R phase reconstruction corresponds to 308 ms, or mid systole, whereas at a heat rate of 57 bpm (average R–R cycle length of 1,053 ms), a reconstruction interval for evaluation of the RCA, placed at 40 % of the R–R cycle displaces to a 420 ms absolute delay (i.e. end-systole) (Fig. 5 and Table 4) Therefore when using traditional relative phase percentage reconstructions the specified period of the cardiac cycle is highly variable at differing heart rates.
Fig. 5.
Reconstruction interval for evaluation of the RCA, placed at 40 % of the R–R cycle for HR = 78 and 57 bpm. Velocity maps of two different patients’ RCAs using relative (top, % R–R x-axis), and absolute (bottom, ms x-axis) demonstrate differences in minimal systolic velocities and their variable definitions using the two methods. Note that despite highly disparate heart rates, the minimal velocity time point lies similarly close to 400 ms after the R-wave despite nearly 10 % difference between the relative portion of the R–R intervals
Table 4.
RCA reconstruction interval at 10 % phase increment for HR = 78 and 57 bpm
| HR = 78 bpm phases (%) | Time (ms) | Velocity (mm/s) |
|
| ||
| 10 | 77 | 60.2 |
| 20 | 154 | 28.9 |
| 30 | 231 | 36.2 |
| 40 | 308 | 32.2 |
| 50 | 385 | 14.4 |
| 60 | 462 | 41.8 |
| 70 | 539 | 18.9 |
| 80 | 616 | 28.2 |
| 90 | 693 | 45.1 |
|
| ||
| HR = 57 bpm phases (%) | Time (ms) | Velocity (mm/s) |
|
| ||
| 10 | 105 | 22.6 |
| 20 | 210 | 79.8 |
| 30 | 315 | 32.0 |
| 40 | 420 | 7.9 |
| 50 | 525 | 32.5 |
| 60 | 630 | 38.1 |
| 70 | 735 | 26.3 |
| 80 | 840 | 14.0 |
| 90 | 945 | 22.6 |
In our study the image reconstructions performed using an absolute delay resulted in selected phases with good or excellent image quality in all patients, as clinically deemed and reported. Thus all selected coronary artery landmarks could be visualized and their location precisely analyzed, albeit at slightly differing time points. Accordingly, we calculated the optimal velocities of the selected coronary arteries and examined the motions’ heart rate dependency. We found no significant correlation between the heart rate and coronary artery motion velocities, except for the AM1 branch.
To our knowledge, no other previous studies have investigated the image quality during the end-systolic temporal window by using absolute delay image reconstruction based on the coronary artery motion in patients with different heart rates.
We believe our findings have three applications in the current era of cardiac CT. First, as prior work has established, systolic targets are highly useful in the setting of tachycardia and arrhythmia, in order to salvage diagnostic coronary CTA [10]. Second, as described in the multimodality imaging guideline for aortic valve intervention, evaluation can be improved by systolic absolute-delay reconstructions, of particular importance given the now well-established role of cardiac CT for percutaneous valve interventions planning [32]. Third, the field of myocardial stress perfusion CT is emerging, and image acquisitions are performed during the administration of pharmacologic vasodilator stress agents; these agents raise heart rates, often shortening or eliminating diastolic windows for acquisition. Because acute beta-blockade has been shown to decrease the efficacy of pharmacologic stress, the ability to image in systole may be a key element to the performance of stress perfusion CT, which is technically challenging and depends upon concomitant coronary artery imaging [33, 34].
Our study does have limitations. The study has a small cohort of 21 patients, however it is sufficient number to give 1488 landmark points for velocity evaluation. Ours is a single center and a single vendor study. We used a second-generation dual source scanner which gave temporal resolution of 75 ms which is relatively low as compared to commonly available single-source scanners; since our data was acquired, native temporal resolutions have been decreased to 66 ms with more modern scanners. Motion could also be confounded by respiratory motion artifact, which is very difficult to subtract from the final image. Lastly, our vendor’s definition of a phase was the “phase start”; whereas other vendors may define a phase reconstruction by the “phase center” and this careful distinction is important if generalizing our findings to other systems.
In conclusion, during an absolute delay of 200–420 ms after the R-wave, the ideal reconstruction interval varies significantly among coronary artery segments. Decreased velocities occur between 280 to 340 ms. Therefore a narrow range of systolic intervals, rather than a single phase, should be acquired.
Footnotes
Compliance with ethical standards
Conflict of interest None.
References
- 1.Achenbach S, Moshage W, Ropers D, Nossen J, Daniel WG. Value of electron-beam computed tomography for the noninvasive detection of high-grade coronary-artery stenoses and occlusions. N Engl J Med. 1998;339:1964–1971. doi: 10.1056/NEJM199812313392702. [DOI] [PubMed] [Google Scholar]
- 2.Reddy GP, Chernoff DM, Adams JR, Higgins CB. Coronary artery stenoses: assessment with contrast-enhanced electron-beam CT and axial reconstructions. Radiology. 1998;208:167–172. doi: 10.1148/radiology.208.1.9646809. [DOI] [PubMed] [Google Scholar]
- 3.Little WC, Downes TR, Applegate RJ. Invasive evaluation of left ventricular diastolic performance. Herz. 1990;15:362–376. [PubMed] [Google Scholar]
- 4.Leschka S, Husmann L, Desbiolles LM, et al. Optimal image reconstruction intervals for non-invasive coronary angiography with 64-slice CT. Eur Radiol. 2006;16:1964–1972. doi: 10.1007/s00330-006-0262-x. [DOI] [PubMed] [Google Scholar]
- 5.Bley TA, Ghanem NA, Foell D, et al. Computed tomography coronary angiography with 370-millisecond gantry rotation time: evaluation of the best image reconstruction interval. J Comput Assist Tomogr. 2005;29:1–5. doi: 10.1097/01.rct.0000149234.09647.8c. [DOI] [PubMed] [Google Scholar]
- 6.Chung CS, Karamanoglu M, Kovacs SJ. Duration of diastole and its phases as a function of heart rate during supine bicycle exercise. Am J Physiol Heart Circ Physiol. 2004;287:H2003–H2008. doi: 10.1152/ajpheart.00404.2004. [DOI] [PubMed] [Google Scholar]
- 7.Mahabadi AA, Achenbach S, Burgstahler C, et al. Safety, efficacy, and indications of beta-adrenergic receptor blockade to reduce heart rate prior to coronary CT angiography. Radiology. 2010;257:614–623. doi: 10.1148/radiol.10100140. [DOI] [PubMed] [Google Scholar]
- 8.Gharib AM, Herzka DA, Ustun AO, et al. Coronary MR angiography at 3T during diastole and systole. J Magn Reson Imaging. 2007;26:921–926. doi: 10.1002/jmri.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shin T, Pohost GM, Nayak KS. Systolic 3D first-pass myocardial perfusion MRI: Comparison with diastolic imaging in healthy subjects. Magn Reson Med. 2010;63:858–864. doi: 10.1002/mrm.22315. [DOI] [PubMed] [Google Scholar]
- 10.Seifarth H, Wienbeck S, Pusken M, et al. Optimal systolic and diastolic reconstruction windows for coronary CT angiography using dual-source CT. AJR Am J Roentgenol. 2007;189:1317–1323. doi: 10.2214/AJR.07.2711. [DOI] [PubMed] [Google Scholar]
- 11.Herzog C, Abolmaali N, Balzer JO, et al. Heart-rate-adapted image reconstruction in multidetector-row cardiac CT: influence of physiological and technical prerequisite on image quality. Eur Radiol. 2002;12:2670–2678. doi: 10.1007/s00330-002-1553-5. [DOI] [PubMed] [Google Scholar]
- 12.Marwan M, Hausleiter J, Abbara S, et al. Multicenter Evaluation Of Coronary Dual-Source CT angiography in patients with intermediate Risk of Coronary Artery Stenoses (MEDIC): study design and rationale. J Cardiovasc Comput Tomogr. 2014;8:183–188. doi: 10.1016/j.jcct.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Johnson TR, Nikolaou K, Wintersperger BJ, et al. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006;16:1409–1415. doi: 10.1007/s00330-006-0298-y. [DOI] [PubMed] [Google Scholar]
- 14.Lee AM, Beaudoin J, Engel LC, et al. Assessment of image quality and radiation dose of prospectively ECG-triggered adaptive dual-source coronary computed tomography angiography (cCTA) with arrhythmia rejection algorithm in systole versus diastole: a retrospective cohort study. Int J Cardiovasc Imaging. 2013;29:1361–1370. doi: 10.1007/s10554-013-0208-8. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie CJ, Godwin JD, Crawford CR, Stanford W, Anno H, Kim Y. Minimum scan speeds for suppression of motion artifacts in CT. Radiology. 1992;185:37–42. doi: 10.1148/radiology.185.1.1523332. [DOI] [PubMed] [Google Scholar]
- 16.Lee AM, Engel LC, Shah B, et al. Coronary computed tomography angiography during arrhythmia: radiation dose reduction with prospectively ECG-triggered axial and retrospectively ECG-gated helical 128-slice dual-source CT. J Cardiovasc Comput Tomogr. 2012;6(172–183):e2. doi: 10.1016/j.jcct.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Ghoshhajra BB, Engel LC, Karolyi M, et al. Cardiac computed tomography angiography with automatic tube potential selection: effects on radiation dose and image quality. J Thorac Imaging. 2013;28:40–48. doi: 10.1097/RTI.0b013e3182631e8a. [DOI] [PubMed] [Google Scholar]
- 18.Austen WG, Edwards JE, Frye RL, et al. A reporting system on patients evaluated for coronary artery disease. Report of the ad hoc committee for grading of coronary artery disease, council on cardiovascular surgery, American heart association. Circulation. 1975;51:5–40. doi: 10.1161/01.cir.51.4.5. [DOI] [PubMed] [Google Scholar]
- 19.Husmann L, Leschka S, Desbiolles L, et al. Coronary artery motion and cardiac phases: dependency on heart rate—implications for CT image reconstruction. Radiology. 2007;245:567–576. doi: 10.1148/radiol.2451061791. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Vidan E, Bergman GW. Cardiac motion of coronary arteries: variability in the rest period and implications for coronary MR angiography. Radiology. 1999;213:751–758. doi: 10.1148/radiology.213.3.r99dc41751. [DOI] [PubMed] [Google Scholar]
- 21.Boudoulas H, Geleris P, Lewis RP, Rittgers SE. Linear relationship between electrical systole, mechanical systole, and heart rate. Chest. 1981;80:613–617. doi: 10.1378/chest.80.5.613. [DOI] [PubMed] [Google Scholar]
- 22.Adler G, Meille L, Rohnean A, Sigal-Cinqualbre A, Capderou A, Paul JF. Robustness of end-systolic reconstructions in coronary dual-source CT angiography for high heart rate patients. Eur Radiol. 2010;20:1118–1123. doi: 10.1007/s00330-009-1642-9. [DOI] [PubMed] [Google Scholar]
- 23.Kim HY, Lee JW, Hong YJ, et al. Dual-source coronary CT angiography in patients with high heart rates using a prospectively ECG-triggered axial mode at end-systole. Int J Cardiovasc Imaging. 2012;28(Suppl 2):101–107. doi: 10.1007/s10554-012-0142-1. [DOI] [PubMed] [Google Scholar]
- 24.Paul JF, Amato A, Rohnean A. Low-dose coronary-CT angiography using step and shoot at any heart rate: comparison of image quality at systole for high heart rate and diastole for low heart rate with a 128-slice dual-source machine. Int J Cardiovasc Imaging. 2013;29:651–657. doi: 10.1007/s10554-012-0110-9. [DOI] [PubMed] [Google Scholar]
- 25.Okada M, Nakashima Y, Shigemoto Y, et al. Systolic reconstruction in patients with low heart rate using coronary dual-source CT angiography. Eur J Radiol. 2011;80:336–341. doi: 10.1016/j.ejrad.2011.01.104. [DOI] [PubMed] [Google Scholar]
- 26.Hausleiter J, Meyer TS, Martuscelli E, et al. Image quality and radiation exposure with prospectively ECG-triggered axial scanning for coronary CT angiography: the multicenter, multivendor, randomized PROTECTION-III study. JACC Cardiovasc Imaging. 2012;5:484–493. doi: 10.1016/j.jcmg.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Luisada AA, MacCanon DM. The phases of the cardiac cycle. Am Heart J. 1972;83:705–711. doi: 10.1016/0002-8703(72)90412-7. [DOI] [PubMed] [Google Scholar]
- 28.Fabian J, Epstein EJ, Coulshed N. Duration of phases of left ventricular systole using indirect methods, I. Normal subjects. Br Heart J. 1972;34:874–881. doi: 10.1136/hrt.34.9.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mollet NR, Cademartiri F, de Feyter PJ. Non-invasive multislice CT coronary imaging. Heart. 2005;91:401–407. doi: 10.1136/hrt.2004.034009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannu HK, Flohr TG, Corl FM, Fishman EK. Current concepts in multi-detector row CT evaluation of the coronary arteries: principles, techniques, and anatomy. Radiographics. 2003;23(Spec No):S111–25. doi: 10.1148/rg.23si035514. [DOI] [PubMed] [Google Scholar]
- 31.Boehm T, Husmann L, Leschka S, Desbiolles L, Marincek B, Alkadhi H. Image quality of the aortic and mitral valve with CT: relative versus absolute delay reconstruction. Acad Radiol. 2007;14:613–624. doi: 10.1016/j.acra.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Bloomfield GS, Gillam LD, Hahn RT, et al. A practical guide to multimodality imaging of transcatheter aortic valve replacement. JACC Cardiovasc Imaging. 2012;5:441–455. doi: 10.1016/j.jcmg.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Qin L, Shi X, et al. Adenosine-stress dynamic myocardial perfusion imaging with second-generation dual-source CT: comparison with conventional catheter coronary angiography and SPECT nuclear myocardial perfusion imaging. AJR Am J Roentgenol. 2012;198:521–529. doi: 10.2214/AJR.11.7830. [DOI] [PubMed] [Google Scholar]
- 34.Taillefer R, Ahlberg AW, Masood Y, et al. Acute beta-blockade reduces the extent and severity of myocardial perfusion defects with dipyridamole Tc-99 m sestamibi SPECT imaging. J Am Coll Cardiol. 2003;42:1475–1483. doi: 10.1016/s0735-1097(03)01046-5. [DOI] [PubMed] [Google Scholar]