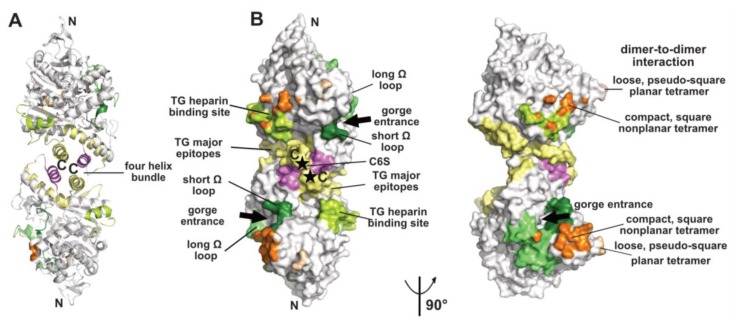
Figure 1.
Overview of a representative α/β-hydrolase fold molecule (mAChE, PDB code 1J06) displayed as a dimer of subunits related by a two-fold symmetry axis, in ribbon (A) and surface modes (B) (with the left and right dimers oriented 90° from each other). The functional surfaces that mediate peptidic ligand binding and tetrameric assembly are color-coded differentially and labeled: helices α3(7,8) and α10 that form the four-helix bundle at the dimer interface are displayed in yellow and violet and the long and short Ω loops that form part of the PAS are in medium green and dark green, respectively. The major epitopes (of which one corresponds to helix α10) and the heparin-binding site in hTG are shown in yellow and light green, respectively, while the attachment sites for chondroitin 6-sulfate oligosaccharide moiety at the C-termini of the subunits are indicated by asterisks and labeled C6S. The surfaces buried at the EeAChE dimer-to-dimer interfaces are shown in wheat for the loose, pseudo-square planar tetramer (modeled from PDB code 1C2B) and in orange for the compact, square nonplanar tetramer (modeled from PDB code 1C2O). The N- and C-termini of each subunit in the dimer and the two active-center gorge entrances are indicated. The nomenclature used for the secondary structure elements is that of [37].