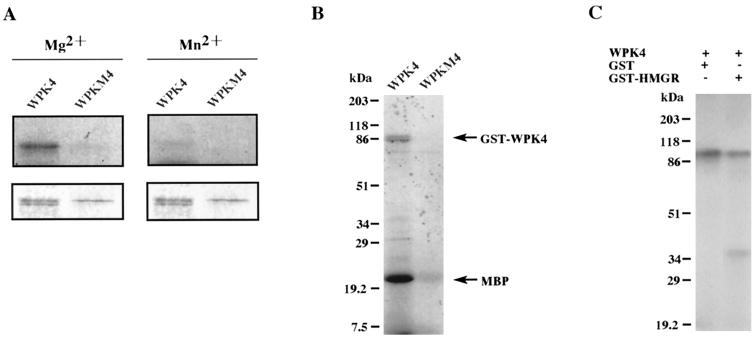
Figure 1.
Phosphorylation analysis of GST-WPK4 and GST-WPKM4 (mutant) fusion proteins. A, Samples were autophosphorylated in reaction mixtures containing either Mg2+ (left) or Mn2+ (right) and separated by SDS-PAGE. The autoradiogram and profiles of the gel stained with Coomassie Brilliant Blue are shown in the top and bottom panels, respectively. B and C, In vitro phosphorylation analysis of GST-WPK4 fusion proteins. Aliquots of 100 ng of GST-WPK4 or GST-WPKM4 fusion proteins were incubated in the presence of 500 ng of myelin basic protein (B), GST (C), or GST-HMGR (C) as the substrates. After the phosphorylation reaction, samples were separated by 12.5% (w/v) SDS-PAGE, dried, and exposed for autoradiography. Relative molecular masses of the standard samples are indicated on the left in kD.