Abstract
Background/Aims
We investigated whether inflammatory markers such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) independently and in combination would be significant prognostic factors for survival in patients with locally advanced pancreatic cancer.
Methods
A total of 497 patients with locally advanced pancreatic cancer who received neoadjuvant or definitive chemoradiotherapy from 2005 to 2015 were evaluated. We divided the patients into groups according to the median values of NLR and PLR: NLR<1.89 (n=156), NLR≥1.89 (n=341), PLR <149 (n=248) and PLR≥149 (n=249).
Results
For NLR <1.89 and ≥1.89 groups, respectively, the 1-year overall survival (OS) rates were 73.2% and 60.8% (p<0.001) and 1-year progression-free survival (PFS) rates were 43.9% and 31.3% (p<0.001). For PLR <149 and ≥149 groups, respectively, the 1-year OS rates were 68.1% and 61.3% (p=0.029) and 1-year PFS rates were 37.9% and 32.5% (p=0.027). Patients with both high NLR and high PLR showed the worst OS and PFS rates compared with those with both lower NLR and lower PLR.
Conclusions
Elevated pretreatment NLR and PLR independently and in combination significantly predicted poor OS and PFS.
Keywords: Pancreatic neoplasms, Prognosis, Neutrophils, Platelet count, Lymphocyte count
INTRODUCTION
Among major solid tumors, pancreatic cancer is well known for its poor prognosis.1 Although surgical treatment is the treatment of choice for pancreatic cancer, only 20% of patients are candidates for curative resection.2 Pancreatic cancer is considered to be resectable when the tumor is not in contact with major arteries (celiac axis, superior mesenteric artery, or common hepatic artery) or major veins (superior mesenteric vein or portal vein). If the patient has locally advanced, such as unresectable or borderline resectable, pancreatic cancer, chemotherapy or definitive or preoperative concurrent chemoradiotherapy (CCRT) is recommended, and the survival is much poorer than those with resectable tumors.3 Thus, it is important to find the prognostic factors for patients with locally advanced pancreatic cancer and set an optimal treatment plan.
Recently, the notion that the outcome of cancer patients is affected not only by tumor characteristics but also by host-related factors has gained much attention. Host factors such as age, sex, performance status, and inflammatory status have been identified as significant prognostic factors in many reports.4–6 As it has become apparent that cancer-associated inflammation is a key determinant of disease progression and survival for cancer patients, there has been much interest in the relationship between patient prognosis and inflammatory hematologic markers.7,8 Among inflammatory hematologic markers, the neutrophil-lymphocyte ratio (NLR) and the platelet-lymphocyte ratio (PLR) are well-known hematologic markers, and studies have suggested that NLR and PLR are associated with the outcome of cancer patients.5,7
Although there are many studies reporting the significance of NLR and PLR as prognostic factors in pancreatic cancer, almost none have shown the efficacy of NLR and PLR in patients with locally advanced pancreatic cancer receiving CCRT.7,9–13 Thus, this study aimed to investigate whether NLR and PLR are associated with the survival outcomes of patients with locally advanced pancreatic cancer treated with CCRT.
MATERIALS AND METHODS
1. Patients
We retrospectively examined the medical records of patients with locally advanced pancreatic cancer who had undergone neoadjuvant or definitive CCRT from January 2005 to December 2015. All patients who had previously received any other treatment, patients who had been diagnosed with a double primary malignancy, patients with distant metastasis at diagnosis, patients who had received RT alone or under 20 Gy, patients with no follow-up imaging, and patients with a neuroendocrine tumor were excluded. Finally, 497 patients were included in the analysis. We retrospectively collected and analyzed patient data, including clinical and laboratory information including white blood cell differential counts and platelet counts. The NLR was calculated by dividing the neutrophil count by the lymphocyte count, and the PLR was calculated by dividing the platelet count by the lymphocyte count. All NLR and PLR values were measured before the start of treatment within 2 weeks. The resectability of pancreatic tumors was determined based on imaging studies including computed tomography (CT) and magnetic resonance imaging (MRI).
The study was approved by the Institutional Review Board of Severance Hospital (IRB No. 2017-3183-01) and performed in accordance with the principles of the Declaration of Helsinki. The informed consent was waived for this retrospective study.
2. Treatment
All patients included in this study received initial preoperative or definitive CCRT.
For RT, CT simulation was performed with intravenous contrast and four-dimensional CT when possible, and respiration training was done to minimize the respiration motion. The primary tumor and involved lymph nodes were set as the gross tumor volume (GTV). The internal target volume (ITV) was delineated when possible, and the planning target volume (PTV) was defined as the GTV or ITV plus a 5-mm margin. A total of 281 patients (56.5%) received three-dimensional conformal RT (3DCRT), and the remaining 216 patients (43.5%) received intensity-modulated radiotherapy (IMRT). The most commonly used dose scheme was 50.4 Gy in 28 patients for 3DCRT patients and 58.42 Gy in 23 fractions for IMRT patients.
For chemotherapy, various chemotherapy regimens were used: (1) 1,000 mg/m2 gemcitabine weekly (days 1, 8, and 15) followed by a 1-week rest period; (2) 75 mg/m2 cisplatin on day 1 of each 28-day cycle combined with weekly 1,000 mg/m2 gemcitabine; (3) 500 mg/m2 fluorouracil weekly; (4) 850 mg/m2 capecitabine twice daily; (5) 40 mg/m2 titanium silicate-1 twice daily from days 1 to 14 and from days 22 to 35; and (6) 0.5 g tegafur/uracil three times daily. The primary aim was to administer full-dose chemotherapy.
3. Response assessment
Routine follow-up was performed at 1 and 3 months after CCRT and routinely after that. The Response Evaluation Criteria in Solid Tumors were used to evaluate the treatment response. The progression of the disease was observed based on imaging studies such as CT or MRI. The recurrence of tumor, increased size of primary tumor, or development of regional or distant metastasis was defined as progression.
4. Statistical analysis
The primary endpoint of our study was overall survival (OS), and the secondary endpoints were progression-free survival (PFS), local failure-free rate (LFFR), and distant failure-free rate (DFFR). All endpoints were calculated from the first date of treatment to the date of the event. The Kaplan-Meier methods and log-rank test were used to calculate the cumulative probabilities of OS, PFS, LFFR, and DFFR. Univariate and multivariate analyses were performed using the Cox proportional hazards model. Multivariate analysis was performed using the statistically significant factors proven using univariate analysis. The SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used for all analyses.
RESULTS
1. Patient and tumor characteristics
Patient and tumor characteristics are shown in Table 1. We divided the patients into groups based on receiver operating characteristics value for the pretreatment NLR and the median value for PLR. The value of NLR and PLR before treatment were 1.89 and 149 respectively. For the two groups divided by the receiver operating characteristics value of NLR, characteristics such as age, sex, Eastern Cooperative Oncology Group (ECOG) status, pathology, tumor stage (T stage), node stage (N stage), chemotherapy regimen, resectability and RT dose did not show significant difference between the two groups. However, there were more patients with tumor size >3.1 cm and less patients with pancreatic head tumor in the higher NLR group. Regarding the two groups divided by the median value of PLR, most patient and tumor characteristics did not differ, except for the subsite and T stage. More patients had a pancreas head tumor in the higher PLR group. However, more patients had T4 stage disease in the lower PLR group.
Table 1.
Patient and Tumor Characteristics
| Characteristic | Total (n=497) | NLR<1.89 (n=156) | NLR≥1.89 (n=341) | p-value | PLR<149 (n=248) | PLR≥149 (n=249) | p-value |
|---|---|---|---|---|---|---|---|
| Age, yr | 64 (35–88) | 62 (35–83) | 64 (35–88) | 0.198 | 63 (35–88) | 64 (35–87) | 0.682 |
| ≤64 | 272 (54.7) | 92 (59.0) | 180 (52.8) | 138 (55.6) | 134 (53.8) | ||
| >64 | 225 (45.3) | 64 (41.0) | 161 (47.2) | 110 (44.4) | 115 (46.2) | ||
| Sex | 0.091 | 0.687 | |||||
| Female | 202 (40.6) | 72 (46.2) | 130 (38.1) | 103 (41.5) | 99 (39.8) | ||
| Male | 295 (59.4) | 84 (53.8) | 211 (61.9) | 145 (58.5) | 150 (60.2) | ||
| ECOG | 0.924* | 0.374* | |||||
| ECOG 0 | 131 (26.4) | 41 (26.3) | 90 (26.4) | 71 (28.6) | 60 (24.1) | ||
| ECOG 1 | 358 (72.0) | 113 (72.4) | 245 (71.8) | 172 (69.4) | 186 (74.7) | ||
| ECOG 2 | 8 (1.6) | 2 (1.3) | 6 (1.8) | 5 (2.0) | 3 (1.2) | ||
| Pathology | 0.157* | 0.517* | |||||
| Adenoca | 413 (83.1) | 135 (86.5) | 278 (81.5) | 209 (84.3) | 204 (81.9) | ||
| Adenosquamous carcinoma | 5 (1.0) | 0 | 5 (1.5) | 3 (1.2) | 2 (0.8) | ||
| Poor differentiated | 5 (1.0) | 0 | 5 (1.5) | 1 (0.4) | 4 (1.6) | ||
| Unknown | 74 (14.9) | 21 (13.5) | 53 (15.5) | 35 (14.1) | 39 (15.7) | ||
| Subsite | 0.014 | 0.003 | |||||
| Head | 345 (69.4) | 120 (76.9) | 225 (66.0) | 157 (63.3) | 188 (75.5) | ||
| Not head | 152 (30.6) | 36 (23.1) | 116 (34.0) | 91 (36.7) | 61 (24.5) | ||
| Clinical T stage | 0.277* | 0.013* | |||||
| T1 | 7 (1.4) | 4 (2.6) | 3 (0.9) | 3 (1.2) | 4 (1.6) | ||
| T2 | 33 (6.6) | 8 (5.1) | 25 (7.3) | 12 (4.8) | 21 (8.4) | ||
| T3 | 204 (41.0) | 69 (44.2) | 135 (39.6) | 89 (35.9) | 115 (46.2) | ||
| T4 | 253 (50.9) | 75 (48.1) | 178 (52.2) | 144 (58.1) | 109 (43.8) | ||
| Clinical N stage | 0.584 | 0.826 | |||||
| N0 | 297 (59.8) | 96 (61.5) | 201 (58.9) | 147 (59.3) | 150 (60.2) | ||
| N1 | 200 (40.2) | 60 (38.5) | 140 (41.1) | 101 (40.7) | 99 (39.8) | ||
| Tumor size, cm | 3.10 (1.20–9.00) | 3.00 (1.20–6.00) | 3.30 (1.20–9.00) | 0.006 | 3.20 (1.20–9.00) | 3.00 (1.20–9.00) | 0.346 |
| ≤3.1 | 251 (50.5) | 93 (59.6) | 158 (46.3) | 120 (48.4) | 131 (52.6) | ||
| >3.1 | 246 (49.5) | 63 (40.4) | 183 (53.7) | 128 (51.6) | 118 (47.4) | ||
| Resectability | 0.132 | 0.049 | |||||
| Unresectable | 378 (76.1) | 112 (71.8) | 266 (78.0) | 198 (79.8) | 180 (72.3) | ||
| Borderline | 119 (23.9) | 44 (28.2) | 75 (22.0) | 50 (20.2) | 69 (27.7) | ||
| Chemotherapy regimen | 0.668 | 0.501 | |||||
| Gemcitabine based | 251 (50.5) | 81 (51.9) | 170 (49.9) | 129 (52.0) | 122 (49.0) | ||
| Not gemcitabine based | 246 (49.5) | 75 (48.1) | 171 (50.1) | 119 (48.0) | 127 (51.0) | ||
| CA 19-9 | 304.5 (0–20,000) | 211.80 (0.10–20,000) | 374.00 (0–20000) | 0.031 | 305.0 (0.10–20,000) | 304.0 (0–20,000) | 0.893 |
| ≤304.5 | 247 (49.9) | 89 (57.1) | 158 (46.6) | 122 (49.6) | 125 (50.2) | ||
| >304.5 | 248 (50.1) | 67 (42.9) | 181 (53.4) | 124 (50.4) | 124 (49.8) | ||
| RT modality | 0.876 | 0.826 | |||||
| 3DCRT | 281 (56.5) | 89 (57.1) | 192 (56.3) | 139 (56.0) | 142 (57.0) | ||
| IMRT+TOMO | 216 (43.5) | 67 (42.9) | 149 (43.7) | 109 (44.0) | 107 (43.0) | ||
| TD EQD2 | 49.56 (24.42–64.31) | 49.56 (38.44–62.00) | 49.56 (24.42–64.31) | 49.56 (24.42–64.31) | 49.56 (42.47–62.00) |
Data are presented median (range) or number (%).
NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio; ECOG, Eastern Cooperative Oncology Group; CA, carbohydrate antigen; RT, radiotherapy; 3DCRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiation therapy; TOMO, tomotherapy; TD, total dose; EQD2, equivalent dose in 2 Gy fractions.
Fisher exact test.
2. Overall survival
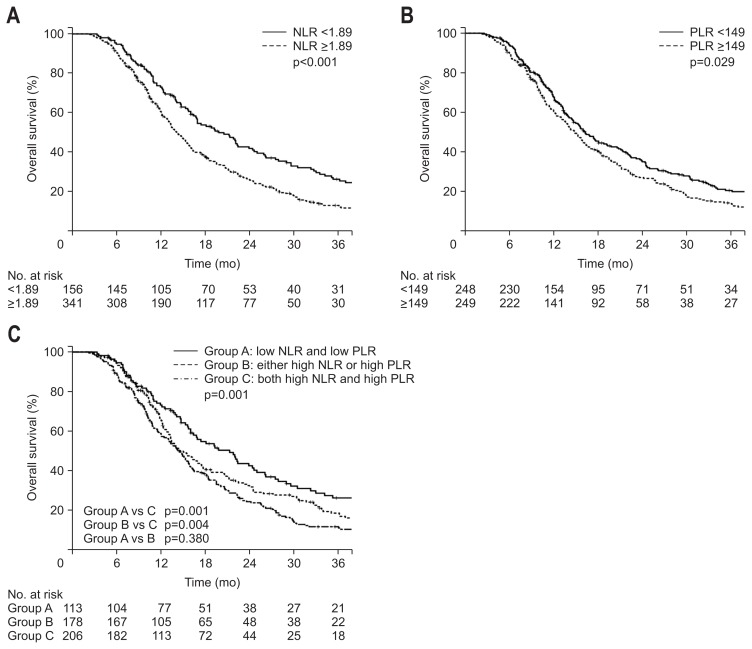
The median follow-up was 19.3 months (range, 4.8 to 128.5 months). The median OS was 15.7 months. The OS was significantly lower in the NLR ≥1.89 group than the NLR <1.89 group (Fig. 1A). The 1-year OS rates were 73.2% and 60.8% for NLR <1.89 and NLR ≥1.89 groups, respectively (p<0.001). The OS also showed a significant difference when the patients were divided by the median PLR value (Fig. 1B). The 1-year OS rates were 68.1% and 61.3% for PLR <149 and PLR ≥149 groups, respectively (p=0.029).
Fig. 1.
Kaplan-Meier estimates of the overall survival according to neutrophil-lymphocyte ratios (NLRs) ≥1.89 or <1.89 (A), platelet-lymphocyte ratios (PLRs) ≥149 or <149 (B), and groups A, B, or C (C).
We divided the patients into three groups according to both the NLR and PLR. Group A included patients with both low NLR and low PLR (n=113), group B included patients with either high NLR or high PLR (n=178), and group C included patients with both high NLR and high PLR (n=206). The 1-year OS rates showed difference between the three groups: 73.3%, 65.9%, and 58.9% for groups A, B, and C, respectively (p=0.001) (Fig. 1C). Although the OS rates were not significantly different between groups A and B, the OS rate of group C was significantly lower than those of groups A and B.
As shown in Tables 2–4, univariate analysis for OS showed that age, sex, ECOG status, tumor size, resectability, CA 19-9 level, NLR, PLR, and grouping of NLR and PLR were significant prognostic factors. Multivariate analysis was also performed and tumor size >3.1 cm (HR, 1.35; 95% CI, 1.09 to 1.66; p=0.005), CA 19-9 >304.5 U/mL (HR, 1.32; 95% CI, 1.08 to 1.61; p=0.006), NLR ≥1.89 (HR, 1.40; 95% CI, 1.12 to 1.75; p=0.003) and PLR ≥149 (HR, 1.35; 95% CI, 1.11 to 1.65; p=0.003) proved to be significant adverse factors for OS. The significant beneficial factors for OS were ECOG score 0 (HR, 0.40; 95% CI, 0.19 to 0.84; p=0.016) and ECOG score 1 (HR, 0.48; 95% CI, 0.24 to 0.98; p=0.046) compared with ECOG score 2 and borderline resectable tumor (HR, 0.62; 95% CI, 0.47 to 0.81; p<0.001). In addition, both lower NLR and lower PLR compared with both higher NLR and higher LR (HR, 0.62; 95% CI, 0.47 to 0.81; p=0.001) and either higher NLR or higher PLR compared with both higher values (HR, 0.73; 95% CI, 0.58 to 0.91; p=0.007) proved to be beneficial factors for OS.
Table 2.
Univariate and Multivariate Analyses of NLR in Overall Survival and Progression-Free Survival
| Overall survival | Progression-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| UVA | MVA | UVA | MVA | |||||||||
|
|
|
|
|
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≤64 vs >64) | 1.26 | 1.04–1.54 | 0.021 | 1.17 | 0.96–1.43 | 0.117 | 1.18 | 0.98–1.43 | 0.08 | |||
| Sex (female vs male) | 1.33 | 1.09–1.63 | 0.006 | 1.25 | 1.03–1.52 | 0.025 | ||||||
| ECOG | 0.038 | 0.026 | 0.559 | |||||||||
| ECOG (ECOG 2 vs 0) | 0.39 | 0.19–0.80 | 0.011 | 0.37 | 0.18–0.78 | 0.009 | 0.7 | 0.34–1.43 | 0.327 | |||
| ECOG (ECOG 2 vs 1) | 0.42 | 0.21–0.85 | 0.016 | 0.44 | 0.21–0.90 | 0.025 | 0.75 | 0.37–1.52 | 0.422 | |||
| Pathology | 0.001 | 0.003 | 0.001 | |||||||||
| Pathology (adenoca vs adenoSCCa) | 0.91 | 0.69–1.19 | 0.494 | 0.83 | 0.63–1.10 | 0.19 | 0.58 | 0.22–1.58 | 0.29 | |||
| Pathology (adenoca vs PD) | 0.73 | 0.27–1.02 | 0.55 | 0.51 | 0.18–1.42 | 0.197 | 5.59 | 2.29–13.64 | <0.001 | |||
| Subsite (head vs other) | 1.16 | 0.94–1.43 | 0.18 | 1.2 | 0.98–1.47 | 0.08 | ||||||
| T stage | 0.051 | 0.47 | ||||||||||
| (T1 vs T2) | 0.34 | 0.13–0.93 | 0.035 | 0.52 | 0.21–1.26 | 0.15 | ||||||
| (T1 vs T3) | 0.99 | 0.67–1.46 | 0.97 | 0.9 | 0.61–1.31 | 0.57 | ||||||
| (T1 vs T4) | 0.81 | 0.66–1.00 | 0.052 | 1.02 | 0.83–1.24 | 0.88 | ||||||
| N stage (N0 vs N1) | 0.99 | 0.81–1.21 | 0.9 | 1.05 | 0.87–1.27 | 0.63 | ||||||
| Size (≤3.1 vs >3.1) | 1.41 | 1.16–1.73 | 0.001 | 1.28 | 1.04–1.57 | 0.02 | 1.4 | 1.16–1.70 | <0.001 | 1.3 | 1.07–1.57 | 0.007 |
| Resectability (unresectable vs borderline) | 0.62 | 0.48–0.80 | <0.001 | 0.65 | 0.50–0.86 | 0.002 | 0.83 | 0.66–1.04 | 0.106 | |||
| Chemotherapy (gemcitabine vs not gemcitabine) | 1.028 | 0.84–1.26 | 0.783 | 1.17 | 0.97–1.42 | 0.098 | ||||||
| CA 19-9 (≤304.5 vs >304.5) | 1.35 | 1.11–1.64 | 0.003 | 1.3 | 1.06–1.59 | 0.009 | 1.38 | 1.14–1.67 | 0.001 | 1.32 | 1.09–1.60 | 0.004 |
| NLR (<1.89 vs ≥1.89) | 1.55 | 1.25–1.93 | <0.001 | 1.4 | 1.12–1.75 | 0.003 | 1.53 | 1.24–1.89 | <0.001 | 1.44 | 1.16–1.78 | 0.001 |
NLR, neutrophil-lymphocyte ratio; UVA, univariate analysis; MVA, multivariate analysis; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SCCa, squamous cell carcinoma; PD, poorly differentiated; CA, carbohydrate antigen.
Table 3.
Univariate and Multivariate Analyses of PLR in Overall Survival and Progression-Free Survival
| Overall survival | Progression-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| UVA | MVA | UVA | MVA | |||||||||
|
|
|
|
|
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≤64 vs >64) | 1.26 | 1.04–1.54 | 0.021 | 1.19 | 0.98–1.46 | 0.076 | 1.18 | 0.98–1.43 | 0.08 | |||
| Sex (female vs male) | 1.33 | 1.09–1.63 | 0.006 | 1.25 | 1.03–1.52 | 0.025 | ||||||
| ECOG | 0.038 | 0.028 | 0.559 | |||||||||
| ECOG (ECOG 2 vs 0) | 0.39 | 0.19–0.80 | 0.011 | 0.38 | 0.18–0.79 | 0.01 | 0.7 | 0.34–1.43 | 0.327 | |||
| ECOG (ECOG 2 vs 1) | 0.42 | 0.21–0.85 | 0.016 | 0.43 | 0.21–0.89 | 0.024 | 0.75 | 0.37–1.52 | 0.422 | |||
| Pathology | 0.001 | 0.002 | 0.001 | |||||||||
| Pathology (adenoca vs adenoSCCa) | 0.91 | 0.69–1.19 | 0.494 | 0.8 | 0.61–1.06 | 0.118 | 0.58 | 0.22–1.58 | 0.29 | |||
| Pathology (adenoca vs PD) | 0.73 | 0.27–1.02 | 0.55 | 0.54 | 0.19–1.51 | 0.241 | 5.59 | 2.29–13.64 | <0.001 | |||
| Subsite (head vs other) | 1.16 | 0.94–1.43 | 0.18 | 1.2 | 0.98–1.47 | 0.08 | ||||||
| T stage | 0.051 | 0.47 | ||||||||||
| (T1 vs T2) | 0.34 | 0.13–0.93 | 0.035 | 0.52 | 0.21–1.26 | 0.15 | ||||||
| (T1 vs T3) | 0.99 | 0.67–1.46 | 0.97 | 0.9 | 0.61–1.31 | 0.57 | ||||||
| (T1 vs T4) | 0.81 | 0.66–1.00 | 0.052 | 1.02 | 0.83–1.24 | 0.88 | ||||||
| N stage (N0 vs N1) | 0.99 | 0.81–1.21 | 0.9 | 1.05 | 0.87–1.27 | 0.63 | ||||||
| Size (≤3.1 vs >3.1) | 1.41 | 1.16–1.73 | 0.001 | 1.38 | 1.12–1.70 | 0.003 | 1.4 | 1.16–1.70 | <0.001 | 1.41 | 1.16–1.71 | <0.001 |
| Resectability (unresectable vs borderline) | 0.62 | 0.48–0.80 | <0.001 | 0.61 | 0.46–0.80 | <0.001 | 0.83 | 0.66–1.04 | 0.106 | |||
| Chemotherapy (gemcitabine vs not gemcitabine) | 1.028 | 0.84–1.26 | 0.783 | 1.17 | 0.97–1.42 | 0.098 | ||||||
| CA 19-9 (≤304.5 vs >304.5) | 1.35 | 1.11–1.64 | 0.003 | 1.32 | 1.08–1.61 | 0.006 | 1.38 | 1.14–1.67 | 0.001 | 1.34 | 1.10–1.61 | 0.003 |
| PLR (<149 vs ≥149) | 1.24 | 1.02–1.50 | 0.029 | 1.35 | 1.11–1.65 | 0.003 | 1.24 | 1.02–1.49 | 0.028 | 1.28 | 1.06–1.56 | 0.009 |
PLR, platelet-lymphocyte ratio; UVA, univariate analysis; MVA, multivariate analysis; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SCCa, squamous cell carcinoma; PD, poorly differentiated; CA, carbohydrate antigen.
Table 4.
Univariate and Multivariate Analyses of NLR and PLR in Overall Survival and Progression-Free Survival
| Overall survival | Progression-free survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| UVA | MVA | UVA | MVA | |||||||||
|
|
|
|
|
|||||||||
| HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≤64 vs >64) | 1.26 | 1.04–1.54 | 0.021 | 1.19 | 0.97–1.45 | 0.09 | 1.18 | 0.98–1.43 | 0.08 | |||
| Sex (female vs male) | 1.33 | 1.09–1.63 | 0.006 | 1.25 | 1.03–1.52 | 0.025 | ||||||
| ECOG | 0.038 | 0.033 | 0.559 | |||||||||
| ECOG (ECOG 2 vs 0) | 0.39 | 0.19–0.80 | 0.011 | 0.4 | 0.19–0.84 | 0.016 | 0.7 | 0.34–1.43 | 0.327 | |||
| ECOG (ECOG 2 vs 1) | 0.42 | 0.21–0.85 | 0.016 | 0.48 | 0.24–0.98 | 0.046 | 0.75 | 0.37–1.52 | 0.422 | |||
| Pathology | 0.001 | 0.002 | 0.001 | |||||||||
| Pathology (adenoca vs adenoSCCa) | 0.91 | 0.69–1.19 | 0.494 | 0.8 | 0.61–1.06 | 0.129 | 0.58 | 0.22–1.58 | 0.29 | |||
| Pathology (adenoca vs PD) | 0.73 | 0.27–1.02 | 0.55 | 0.51 | 0.18–1.43 | 0.203 | 5.59 | 2.29–13.64 | <0.001 | |||
| Subsite (head vs other) | 1.16 | 0.94–1.43 | 0.18 | 1.2 | 0.98–1.47 | 0.08 | ||||||
| T stage | 0.051 | 0.47 | ||||||||||
| (T1 vs T2) | 0.34 | 0.13–0.93 | 0.035 | 0.52 | 0.21–1.26 | 0.15 | ||||||
| (T1 vs T3) | 0.99 | 0.67–1.46 | 0.97 | 0.9 | 0.61–1.31 | 0.57 | ||||||
| (T1 vs T4) | 0.81 | 0.66–1.00 | 0.052 | 1.02 | 0.83–1.24 | 0.88 | ||||||
| N stage (N0 vs N1) | 0.99 | 0.81–1.21 | 0.9 | 1.05 | 0.87–1.27 | 0.63 | ||||||
| Size (≤3.1 vs >3.1) | 1.41 | 1.16–1.73 | 0.001 | 1.35 | 1.09–1.66 | 0.005 | 1.4 | 1.16–1.70 | <0.001 | 1.39 | 1.15–1.69 | 0.001 |
| Resectability (unresectable vs borderline) | 0.62 | 0.48–0.80 | <0.001 | 0.62 | 0.47–0.81 | 0.001 | 0.83 | 0.66–1.04 | 0.106 | |||
| Chemotherapy (gemcitabine vs not gemcitabine) | 1.03 | 0.84–1.26 | 0.783 | 1.17 | 0.97–1.42 | 0.098 | ||||||
| CA 19-9 (≤304.5 vs >304.5) | 1.35 | 1.11–1.64 | 0.003 | 1.32 | 1.08–1.61 | 0.006 | 1.38 | 1.14–1.67 | 0.001 | 1.35 | 1.11–1.63 | 0.002 |
| NLR and PLR group | 0.001 | 0.001 | <0.001 | <0.001 | ||||||||
| Group C vs group A | 0.60 | 0.46–0.79 | <0.001 | 0.62 | 0.47–0.81 | 0.001 | 0.61 | 0.47–0.79 | <0.001 | 0.63 | 0.49–0.82 | 0.001 |
| Group C vs group B | 0.82 | 0.65–1.02 | 0.072 | 0.73 | 0.58–0.91 | 0.007 | 0.75 | 0.61–0.93 | 0.009 | 0.69 | 0.56–0.86 | 0.001 |
NLR, neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; UVA, univariate analysis; MVA, multivariate analysis; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; SCCa, squamous cell carcinoma; PD, poorly differentiated.
We additionally analyzed the 1-year OS rates of different subgroups. Overall, 105 patients (21.1%) had undergone surgery after CCRT. Fifty-two patients (13.8%) in the unresectable group and 53 (44.5%) in the borderline resectable group had undergone surgery. The 1-year OS rates were 87.4% for the surgery group and 58.2% for the non-surgery group, respectively (p<0.001).
3. Progression-free survival
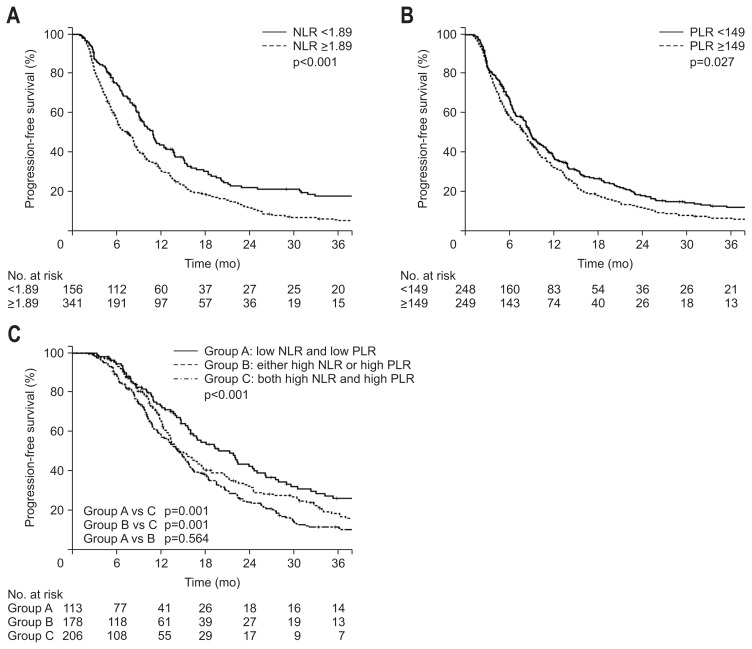
The PFS showed a significant difference between the groups divided by NLR and PLR values. The 1-year PFS rates were 43.9% and 31.3% for NLR <1.89 and NLR ≥1.89 groups, respectively (p<0.001) (Fig. 2A). For PLR, the 1-year PFS rates were 37.9% and 32.5% for PLR <149 and PLR ≥149 groups, respectively (p=0.027) (Fig. 2B). We also examined the PFS for groups A, B, and C, with similar results as those for OS. The 1-year PFS rates for groups A, B, and C were 41.7%, 38.5%, and 29%, respectively (p<0.001) (Fig. 2C). Although there was no significant difference in PFS between groups A and B, the PFS rate of group C was significantly lower than those of groups A and B.
Fig. 2.
Kaplan-Meier estimates of progression-free survival according to neutrophil-lymphocyte ratios (NLRs) ≥1.89 or <1.89 (A), platelet-lymphocyte ratios (PLRs) ≥149 or <149 (B), and groups A, B, or C (C).
On univariate analysis, sex, tumor size, CA 19-9 level, NLR, PLR, and grouping of NLR and PLR proved to be significant prognostic factors for PFS (Table 2–4). Among these factors, tumor size >3.1 cm (HR, 1.39; 95% CI, 1.15 to 1.69; p=0.001), CA19-9 >304.5 U/mL (HR, 1.35; 95% CI, 1.11 to 1.63; p=0.002), NLR ≥1.89 (HR, 1.44; 95% CI, 1.16 to 1.78; p=0.001), and PLR ≥149 (HR, 1.28; 95% CI, 1.06 to 1.56; p=0.009) proved to be significant adverse factors for PFS. Significant favorable factors for PFS was both lower NLR and lower PLR compared with both higher NLR and higher PLR (HR, 0.63; 95% CI, 0.49 to 0.982; p=0.001) and either higher NLR or higher PLR compared with both higher values (HR, 0.69; 95% CI, 0.56 to 0.86; p=0.001).
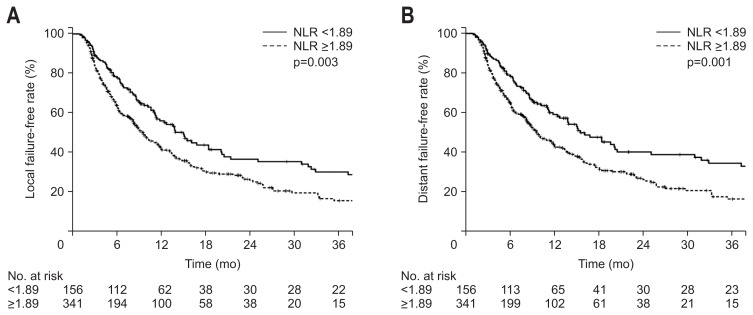
4. Local failure-free rate and distant failure-free rate
When the patients were divided into two groups according to NLR, there was a significant difference for both LFFR and DFFR. The 1-year LFFR was 56.9% and 42.4% (p=0.03) and the 1-year DFFR was 539.2% and 44.0% (p=0.001) for NLR <1.89 and NLR ≥1.89 groups, respectively (Fig. 3).
Fig. 3.
Kaplan-Meier estimates of local failure-free rate (A) and distant failure-free rate (B) according to neutrophil-lymphocyte ratios (NLRs) ≥1.89 or <1.89.
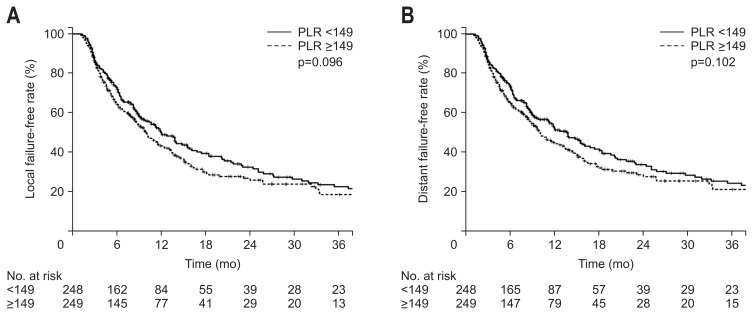
When the patients were divided by a PLR value of 149, there was no significant difference in either LFFR or DFFR. The 1-year LFFR was 50.2% and 43.4% (p=0.096) and the 1-year DFFR was 52.7% and 45.1% (p=0.102) for PLR <149 and PLR ≥149 groups, respectively (Fig. 4).
Fig. 4.
Kaplan-Meier estimates of local failure-free rate (A) and distant failure-free rate (B) according to platelet-lymphocyte ratios (PLRs) ≥149 or <149.
DISCUSSION
It is well known that tumor characteristics such as tumor size, tumor stage, and CA 19-9 level are considered meaningful factors to predict a pancreatic cancer patient’s prognosis.14,15 Recently, other studies have suggested that inflammation is associated with a poor prognosis in cancer patients.16,17 This study aimed to investigate the role of NLR and PLR in predicting the prognosis of patients with locally advanced pancreatic cancer who had undergone preoperative or definitive CCRT. Our results showed that patients with high NLR or PLR showed worse OS and PFS than those with lower NLR or PLR. When both values were taken into consideration, the group with both high NLR and high PLR showed the worst OS and PFS.
Many previous reports have shown the prognostic value of NLR and PLR and their significance as prognostic markers is becoming increasingly important. For example, a study with 403 advanced pancreatic cancer patients reported that NLR ≥3.1 was a significant adverse factor for OS.18 Another study showed that NLR ≥3.3 was a negative factor for 67 elderly patients with un-resectable pancreatic cancer, and other studies have shown NLR >5, NLR ≥2.551, and NLR ≥2.0 as significant prognostic values for OS.7,9,11,19–21 PLR ≥126 was an important prognostic factor in a study involving 321 locally advanced and metastatic pancreatic cancer patients.4 Other studies have shown PLR ≥208.1, PLR ≥200, and PLR >150 to be significant prognostic markers.7,9,13 Although reports concerning PLR are less common in pancreatic cancer than those relating to NLR, it has been reported to be a prognostic factor in many other malignancies.22,23 In our study, both NLR and PLR proved to be a useful prognostic factor for both OS and PFS.
Currently, there is no consensus on the cutoff value of NLR and PLR, and thus, various cutoff values were used in previous studies. In some studies, the receiver operating characteristic curve was used for determining the cutoff value, and in some studies, median values were used.4,9,18,21,24 Some other studies have used cutoff values of 5 for NLR and 150 for PLR.13,19,25,26 Our study set the cutoff values of NLR and PLR using their median values.
In this study, we additionally analyzed OS and PFS taking both NLR and PLR into consideration. The group of patients with both high NLR and high PLR showed the worst OS and PFS. To the best of our knowledge, this is the first study to incorporate both values into the analysis, and these results may aid in adequately determining the prognosis in patients with locally advanced pancreatic cancer.
Currently, the mechanisms responsible for high NLR and PLR being related to poor outcomes of cancer patients are not entirely understood. What is known is that inflammation status promotes tumor proliferation and helps the survival of the malignant tumor.27 Furthermore, factors related to inflammation stimulate angiogenesis of the malignant tumor, invasion, and metastasis.28 As NLR and PLR represent the inflammation status of the host, a high NLR and PLR can be related to a poor prognosis for cancer patients. A high NLR represents both an elevated level of neutrophils and a decreased level of lymphocytes. Lymphocytes are known to mediate the anti-tumor response and neutrophils are known to release cytokines, such as interleukins-1 and -6, and tumor necrosis factor, which promote cancer growth, angiogenesis, and metastasis.29 Therefore, a high NLR, in which a decreased lymphocyte count can be connected to a decreased immune response, and an elevated neutrophil count, which can be related to tumor progression, has the potential to be a significant adverse prognostic factor for cancer patients.
There are several limitations in our study. First, as this is a retrospective study, the heterogeneity of the patients and treatment may have affected the results. Second, although neutrophil and lymphocyte counts can be influenced by infection, drugs, or other factors, we did not consider these factors, which may have biased the results. However, beyond these limitations, this study also has several strengths. Although there are many reports on inflammation and prognosis, the number of patients included in this study is one of the largest in a single institution. In addition, only the patients who received preoperative or definitive CCRT as initial treatment in our institution were included, which may have reduced some bias. Furthermore, NLR or PLR can be easily measured using peripheral blood samples, which is excellent for its usefulness as a prognostic marker.
In summary, this study showed that higher NLR or PLR is related to poor OS and PFS and high NLR is related to poor LFFR and DFFR. Furthermore, when both NLR and PLR values were high, the results were the worst for OS and PFS. Based on these results, parameters showing systemic inflammation such as NLR and PLR can be useful markers for predicting the prognosis for advanced pancreatic cancer patients. Further studies will be needed to validate the usefulness and effectiveness of these inflammatory markers in advanced pancreatic cancer patients.
Footnotes
See editorial on page 223.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. BMJ. 2012;344:e2476. doi: 10.1136/bmj.e2476. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 4.Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145–150. doi: 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 6.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 7.Martin HL, Ohara K, Kiberu A, Van Hagen T, Davidson A, Khattak MA. Prognostic value of systemic inflammation-based markers in advanced pancreatic cancer. Intern Med J. 2014;44:676–682. doi: 10.1111/imj.12453. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 9.Goh BK, Tan DM, Chan CY, et al. Are preoperative blood neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios useful in predicting malignancy in surgically-treated mucin-producing pancreatic cystic neoplasms? J Surg Oncol. 2015;112:366–371. doi: 10.1002/jso.23997. [DOI] [PubMed] [Google Scholar]
- 10.Spolverato G, Maqsood H, Kim Y, et al. Neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after resection for hepato-pancreatico-biliary malignancies. J Surg Oncol. 2015;111:868–874. doi: 10.1002/jso.23900. [DOI] [PubMed] [Google Scholar]
- 11.Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: a retrospective study in a single institute in Japan. Jpn J Clin Oncol. 2015;45:61–66. doi: 10.1093/jjco/hyu159. [DOI] [PubMed] [Google Scholar]
- 12.Stotz M, Gerger A, Eisner F, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith RA, Bosonnet L, Raraty M, et al. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–472. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Desmond RA, Wilcox CM, et al. Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg. 2006;192:322–329. doi: 10.1016/j.amjsurg.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Humphris JL, Chang DK, Johns AL, et al. The prognostic and predictive value of serum CA19.9 in pancreatic cancer. Ann Oncol. 2012;23:1713–1722. doi: 10.1093/annonc/mdr561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishi Y, Kopetz S, Chun YS, Palavecino M, Abdalla EK, Vauthey JN. Blood neutrophil-to-lymphocyte ratio predicts survival in patients with colorectal liver metastases treated with systemic chemotherapy. Ann Surg Oncol. 2009;16:614–622. doi: 10.1245/s10434-008-0267-6. [DOI] [PubMed] [Google Scholar]
- 17.Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 18.Luo G, Guo M, Liu Z, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee JM, Lee HS, Hyun JJ, et al. Prognostic value of inflammation-based markers in patients with pancreatic cancer administered gemcitabine and erlotinib. World J Gastrointest Oncol. 2016;8:555–562. doi: 10.4251/wjgo.v8.i7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 21.Kadokura M, Ishida Y, Tatsumi A, et al. Performance status and neutrophil-lymphocyte ratio are important prognostic factors in elderly patients with unresectable pancreatic cancer. J Gastrointest Oncol. 2016;7:982–988. doi: 10.21037/jgo.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Emic N, Engelman A, Molitoris J, et al. Prognostic significance of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in patients treated with selective internal radiation therapy. J Gastrointest Oncol. 2016;7:269–277. doi: 10.3978/j.issn.2078-6891.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaverdian N, Veruttipong D, Wang J, Schaue D, Kupelian P, Lee P. Pretreatment immune parameters predict for overall survival and toxicity in early-stage non-small-cell lung cancer patients treated with stereotactic body radiation therapy. Clin Lung Cancer. 2016;17:39–46. doi: 10.1016/j.cllc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alagappan M, Pollom EL, von Eyben R, et al. Albumin and neutrophil-lymphocyte ratio (NLR) predict survival in patients with pancreatic adenocarcinoma treated with SBRT. Am J Clin Oncol. 2018;41:242–247. doi: 10.1097/COC.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 26.Bertuzzo VR, Cescon M, Ravaioli M, et al. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279–1285. doi: 10.1097/TP.0b013e3182187cf0. [DOI] [PubMed] [Google Scholar]
- 27.Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shamamian P, Schwartz JD, Pocock BJ, et al. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: a role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 29.Di Carlo E, Forni G, Musiani P. Neutrophils in the antitumoral immune response. Chem Immunol Allergy. 2003;83:182–203. doi: 10.1159/000071561. [DOI] [PubMed] [Google Scholar]