Figure 5.
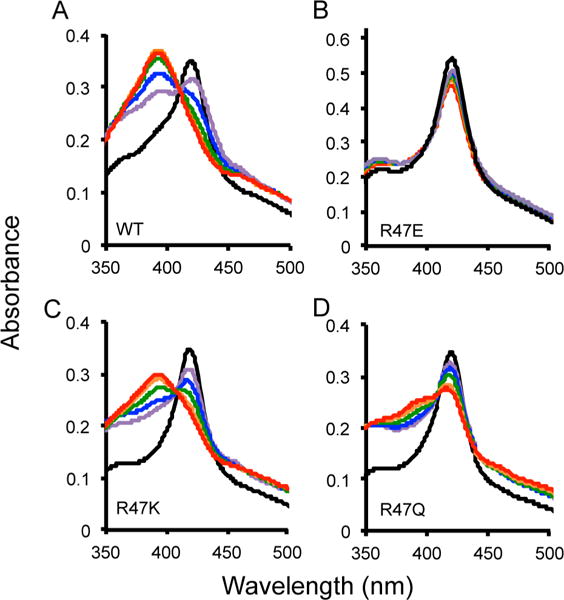
Spin state temperature dependence for BM-3 wild type (A), R47E (B), R47K (C), and R47Q (D). The optical absorbance spectra with a 30-fold excess of NPG was collected at temperatures of 37 (red), 27 (orange), 17 (green), 7 (blue), and −3 °C (purple). The resting state of the enzyme without NPG is shown in black at room temperature. A temperature-dependent change in the spin state of the heme iron was observed with excess NPG because the absorbance at 418 nm (low spin) decreased and at 393 nm (high spin) increased as the temperature increased for wild-type, R47K, and R47Q. There was no temperature-dependent spin state change observed for R47E BM-3 with NPG bound. The percentage of the population in high spin at 37 °C is 97% for the wild-type, 80% for R47K, 50% for R47Q, and 8% for R47E.
