Abstract
We have developed 7-diethylaminocoumarin-based chromophores that photoisomerize with visible light. These photoswitches possess many desirable attributes, including large extinction coefficients (18,600 – 59,100 M−1 cm−1), high quantum yields (0.45 – 0.50) and resistance to photofatigue. Additionally, time-resolved spectroscopy indicates that both isomerization reactions are complete in less than 1 ns.
Graphical abstract
Photochemical transformations are of fundamental scientific importance, with photosynthesis1,2, visual transduction3, time-resolved chemical spectroscopy4, optical recording5, and super-resolution fluorescence imaging6 exemplifying such prominence. Many of these transformations involve photoswitches3,5,6, i.e. molecules that undergo bidirectional, non-destructive structural changes. For many purposes, the ideal photoswitch should efficiently absorb light and undergo fast isomerization, leading to a thermally stable photostationary state (PSS) with high isomeric purity7. These compounds should be photochemically robust enough such that they will exhibit minimal fatigue over the course of many cycles of switching. Additionally, the ability to use visible light to control these systems can be quite useful8,9, especially for biological applications where tissue absorbs and is damaged by UV light.
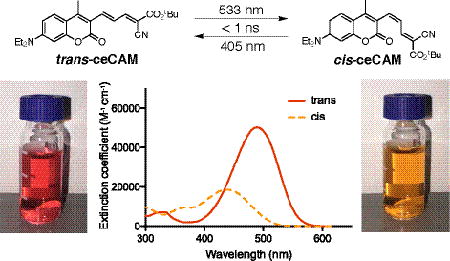
While photoswitchable molecules have been extensively reported7,10,11, most tend to be variations on a few major classes such as azobenzenes12,13, diarylethenes14, spiropyrans15, fulgides16, stilbenes14 and hemithioindigos14. The high molar absorptivity and photostability of many coumarin chromophores such as 7-diethylaminocoumarin17 (DEAC), has led to their extensive use as laser dyes18 and fluorescent probes19, but despite their many photochemical applications the use of coumarins as photochromic materials has been rare and limited mostly to spiropyrans20 and diarylethenes21. We have discovered that attaching strongly electron-withdrawing extended -systems on the 3-position of 4-methyl-DEAC yields highly efficient cis-trans alkene photoswitches. These new coumarin allylidene malonate (CAM) compounds, 1 and 2 (Scheme 1), can be isomerized using visible light (Scheme 1) compatible with modern confocal microscopes for both reactions (e.g. 405 nm and 533 nm) to yield PSSs of 71–96% that are essentially thermally stable for many days. Using ultrafast time-resolved absorption spectroscopy in the 0.1 ps – 2 ns time domain, we have demonstrated rapid and efficient isomerization in both directions (isomerization completed in less than 1 ns, with quantum yields of 0.45–0.50). Light is absorbed efficiently by both isomers (molar extinction coefficients 18,600 – 59,100 M−1 cm−1) and causes no fatigue for more than 100 isomerization cycles.
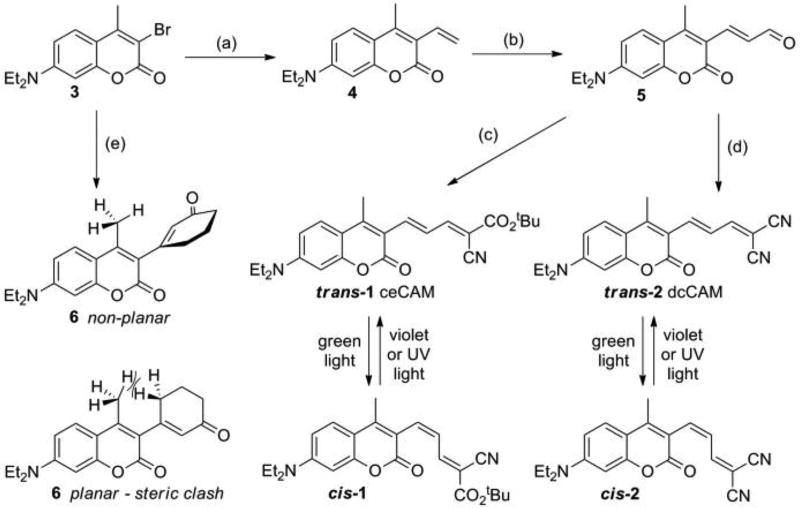
Scheme 1.
Synthesis and isomerization of CAM photoswitches. Reagents and conditions (a) Trivinylboroxin-pyridine complex, Pd(PPh3)4, K2CO3, DME, 85 °C, 18 h, 90% yield; (b) POCl3, DMF, RT, 1 h, 74% yield; (c) tert-Butyl cyanoacetate, piperidine, EtOH, 78 °C, 1 h, 91% yield; (d) Malononitrile, piperidine, EtOH, 78 °C, 1 h, 88% yield; (e) 3-(Boronic acid pinacol ester)-cyclohex-2-enone, PdCl2(dppf), K2CO3, 1,4-dioxane, 101 °C, 2 h, 41% yield.
The synthesis of CAM compounds began with known bromide 317 which was vinylated via a Suzuki cross-coupling to give 4 in 90% yield (Scheme 1). Formylation of this compound under Vilsmeier-Haack conditions resulted in a 74% yield of aldehyde 5. A sterically hindered cyanoester coumarin allylidene malonate (ceCAM, 1) was synthesized by a piperidine-catalyzed Knoevenagel condensation of 5 with tert-butyl cyanoacetate in 91% yield. After column chromatography, ceCAM was recrystallized from ethanol, giving a sample with a single peak on analytical HPLC when carefully protected from room light. This compound (trans-ceCAM) has a molar extinction coefficient of 50,300 M−1 cm−1 at 489 nm in MeCN (Fig. 1b). Strong solvatochromism was observed (Fig. S1) with a molar extinction coefficient of 59,000 M−1 cm−1 at 505 nm in DMSO (Fig. S2).
Figure 1.
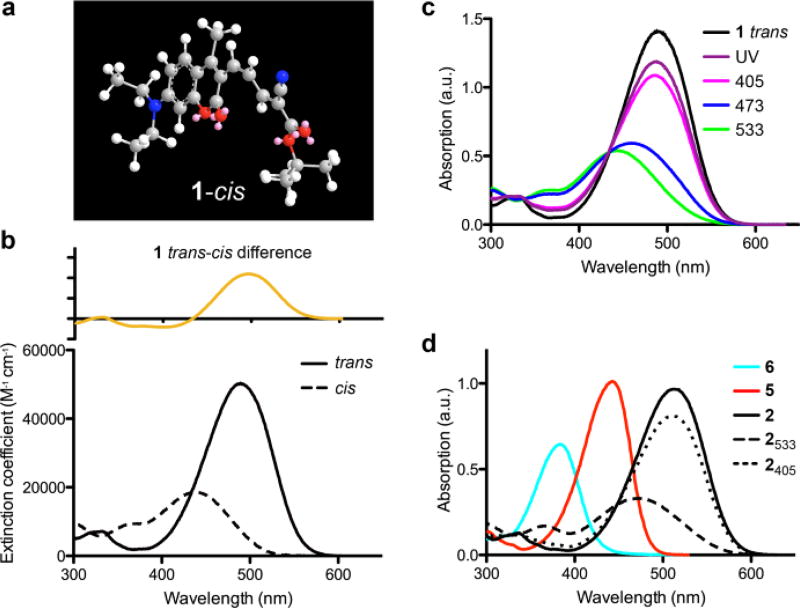
Structure of cis-ceCAM and UV-visible absorption spectra of ceCAM and related compounds. (a) 3D representation of cis-ceCAM derived from MM2 energy minimization calculations in ChemDraw3D. (b) Absorption spectrum of trans-ceCAM (solid) and calculated absorption spectrum of cis-ceCAM (dashed, ε437 = 18,600 M−1 cm−1) in MeCN with difference spectrum (top). (c) PSS of ceCAM in MeCN using various light sources. (d) Absorption spectra in MeCN of aldehyde 5 (red), locked-ketone 6 (cyan) and trans-dcCAM 2 (black) and PSS spectra from 533 nm (dashes) and 405 nm (dots) laser irradiation of dcCAM.
Irradiation of a solution of trans-ceCAM with blue or green light led to a hypsochromic shift in the spectrum which could be reversed with exposure to violet light or UV light (Fig 1c). Such large hypsochromic and hypochromic shifts have not been reported for structurally similar coumarins with extended π-systems that lack a 4-methyl group22. The photochromism observed in ceCAM can be explained by trans/cis photoisomerization (Scheme 1) where the cis isomer is distorted from planarity by the steric clash with the methyl group (Fig. 1a), leading to a loss of conjugation and a shorter λmax. Such a hypsochromic shift caused by the steric twisting of the π-system can be illustrated further with 4-methyl-3-acrylaldehyde-DEAC 5 and its ring-locked analog 6 (Scheme 1). The unhindered aldehyde 5 possesses a λmax at 433 nm and pronounced minimum at 342 nm (Fig. 1d). However, because of the steric clash between the 4-methyl group and the cyclohexenone ring, the absorption spectrum of 6 shows a hypsochromic shift in its λmax (384 nm) with a broader peak and less prominent minimum arising from steric interactions as illustrated in Scheme 1.
To confirm the observed spectral changes arise from conformational change around the double bond directly connected to the 3-position of the coumarin (Fig. 1a), we synthesized the dicyano analog dcCAM (2) as shown in Scheme 1. The redundancy of the terminal nitriles of dcCAM means that only isomerization around the first exocyclic double bond produces a different structure. Thus, the similar spectral changes (Fig. 1d) shown by dcCAM establish that it is this double bond which undergoes trans-cis isomerization in ceCAM (Fig. 1a), with the cis conformation occupying a non-planar structure due to steric interaction with the 4-methyl substituent. Likely because of the more electron-withdrawing character of the malononitrile22, the λmax of trans-dcCAM is even further red-shifted than trans-ceCAM and exhibits higher molar extinction coefficients (trans: ε513 = 59,100 M−1 cm−1; cis: ε462 = 19,100 M−1 cm−1 in MeCN, Fig. S3).
The compositions of the photostationary states of ceCAM were examined using green and near-UV LED lights with emissions centered at 530 and 365 nm, respectively. We found that in MeCN green light gave 88% cis-ceCAM and near-UV light gave 80% trans-ceCAM (Fig. 1c). In DMSO 87% cis-ceCAM and 82% trans-ceCAM were produced, respectively. These ratios were determined by HPLC analysis with reactions monitored at the isosbestic point. Using monochromatic laser light, the PSS compositions for ceCAM in MeCN were found to be 96% cis from 533 nm and 71% trans from 405 nm (Fig. 1b). The isomerization quantum yields were determined using photon counting methods.23,24 A 533-nm laser was used for irradiation of pure trans-1 and the reaction was monitored by HPLC (see Supporting Information, Fig. S4). We found that the trans to cis quantum yield was 0.5. Experimental determination of cis to trans quantum yield using the change in the steady state absorption spectra with time (Fig. S5) produced by irradiation of cis-1 with a filtered 405-nm LED gave a value of 0.45 (see Supporting Information).
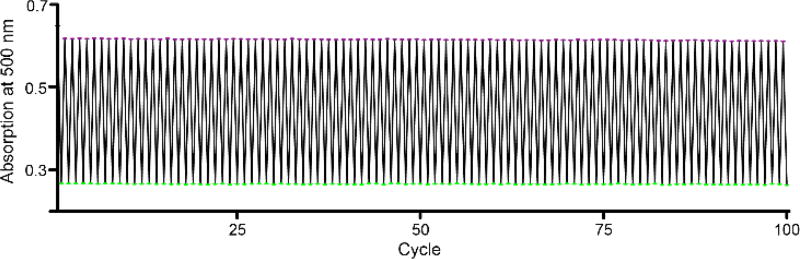
For many applications, thermal bistability of photoswitches is a desirable feature.7,8,13 The stability of the thermodynamically unfavored cis conformation of ceCAM in MeCN solution was monitored by HPLC. After one week, less than 3% of the cis-isomer had converted to the trans-isomer. The thermal isomerization half-life was determined to be 175 days (Fig. S6). In addition to possessing good thermal stability, the ideal photoswitch ought to demonstrate photostability. If photoswitches are to be used long term, such as for data storage5 or photopharmacology25, they must be able to cycle through many rounds of conformational interconversion, ideally without any fatigue of the photochemistry or general photobleaching of the chromophore itself7. We found that 365 nm and 530 nm light could be used to effect 100 conformational interconversions of ceCAM without any noticeable degradation of the absorption spectra (Fig. 2). HPLC analysis did show that repeated interconversion between cis and trans states of ceCAM appears to introduce a small amount of a second trans isomer (Fig. S7), likely from isomerization of the cyanoester-containing terminal double bond. The absorption spectra of these two trans isomers are nearly identical and a second cis isomer was not observed. dcCAM does not develop a similar second isomer (Fig. S8). Finally, we tested the very long term photostability of ceCAM; and found that after 18h we started to detect some photodecomposition from continuous irradiation at 420 nm (Fig. S9). These results suggest that CAM photoswitches could be used in more demanding applications where many repeated cycles of isomerization are required.
Figure 2.
Photofatigue of ceCAM. ceCAM in DMSO was irradiated with light from green and UV LEDs. Light of high flux density (>400 mW cm−2) caused rapid isomerization to the PSS for each wavelength, i.e. 13% and 82% trans-ceCAM. 100 cycles of isomerization produced no detectable fatigue.
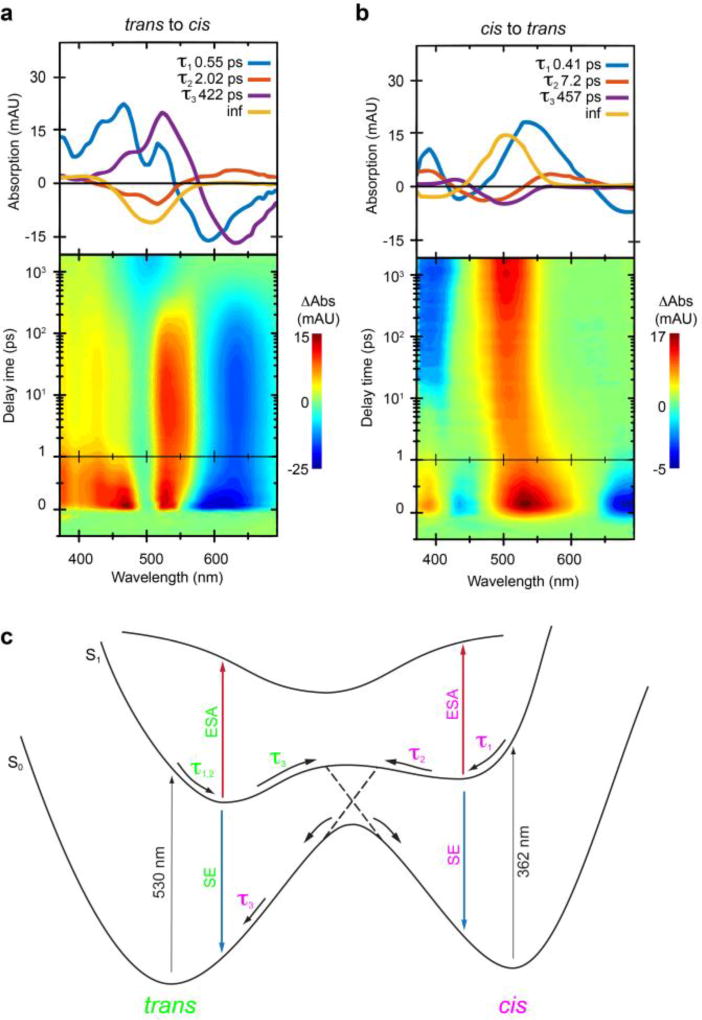
Next, we used time-resolved absorption spectroscopy26 to study the rates of isomerization of ceCAM on an ultrafast timescale. With a time resolution of the system of <150 fs, optical absorption spectra over the entire time course show striking differences for the two isomerization reactions (Fig. 3a,b). In the case of the trans to cis reaction, after excitation at 530 nm two broad excited state absorption (ESA) bands at 390–490 nm and ~525 nm and a stimulated emission (SE) band at ~625 nm appears (Fig. 3a). Global lifetime analysis was performed, and three exponential functions and an infinite lifetime are necessary to describe the experimental data and the residual signal at around 2 ns, respectively. The corresponding decay-associated spectra for the fitted lifetimes are depicted in Fig. 3a (top).
Figure 3.
Kinetics of isomerization. (a,b) Transient absorption spectra were obtained using ultrafast pump-probe spectroscopy for trans – cis and cis – trans isomerization reactions of ceCAM. (c) Potential energy surfaces for both isomerization reactions indicating the place of the respective lifetimes (trans in green, cis in pink) obtained from the global lifetime analysis27 which describe the isomerization pathways. Abbreviations: SE, stimulated emission; ESA, excited state absorption.
The first lifetime, τ1 = 0.55 ps, describes the direct motion from the Franck-Condon region to the relaxed S1 excited state, accompanied by a red/blue-shift of the positive/negative signals on this time range. A Stokes-shift is associated with the second lifetime, τ2 = 2.0 ps, as seen in the decay-associated spectra. This is probably caused by solvent relaxation. The third lifetime, τ3 = 422 ps, describes the decay of the excited state absorption around 525 nm and stimulated emission around 625 nm, both of which are associated with the S1 excited state. The decay of the S1 typically proceeds via a conical intersection and populates the ground state of either the cis- or trans-isomer of ceCAM (Fig. 3c). The residual absorption changes around 400 nm (positive) and 500 nm (negative) match the absorption bands in the steady-state difference spectrum of the isomers, leading to the conclusion that the isomerization is completed within the measurement time of <2 ns.
The transient absorption data for the cis to trans isomerization is strikingly different from the reverse process (Fig. 3b). Initially after photoexcitation at 362 nm four signals appear, consisting of two excited state absorption bands centered around 385 nm and 525 nm and two stimulated emission bands 440 nm and 696 nm. Similar to the trans to cis isomerization, the first lifetime τ1 = 0.41 ps describes the motion away from the Franck-Condon region. Interestingly, the S1 lifetime appears to be comparatively short and decays with τ2 = 7.2 ps, describing the ground state population of either the trans- or cis-isomer. The spectra at these specific delay times resemble the difference steady-state spectra and thus suggest a completed isomerization reaction. Subsequently, a relatively slow signal with τ3 = 457 ps is observed, that leads to a small spectral red-shift of the product band to yield the final steady-state difference spectrum. Overall the data show that the isomerization reactions in both directions are completed in less than 1 ns. If photoswitches are to be used to initiate fast biological processes, the photochemistry must be significantly faster than the biology itself. Our detailed photophysics of the ceCAM chromophore shows that switching is fast enough for such purposes.
We have extensively characterized two new coumarin allylidene malonate (CAM) photoswitches that have a uniquely powerful set of chemical properties (Table S1). Large molar extinction coefficients and good quantum yields result in highly efficient photoswitching. The switches exhibit good thermal stability and are very resistant to photofatigue. Sub-nanosecond isomerization lifetimes make CAM switches potentially quite useful for biological applications. These switches also possess a number of sites that could be chemically modified for various purposes. The dicyano version CAM switch, dcCAM, has two chemically addressable positions on the coumarin side of the double bond (the 4-methyl and the 7-diethylamine), while the ester on ceCAM presents an additional functional handle. These anchor points introduce a nice synthetic flexibility into our new photoswitch for multifarious applications. Considering these attributes, CAM photoswitches could find many uses in materials and biological sciences.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (USA) to GCRE-D, and the Deutsche Forschungsgemeinschaft (DFG) through the research training group GRK 1986 (“CLiC”, Complex scenarios of light-control) to JW.
Footnotes
Electronic Supplementary Information (ESI) available: Supplemental figures, experimental details and analytical data (PDF). See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
References
- 1.Calvin M. Angew. Chem. Int. Ed. 1962;1:65–75. [Google Scholar]
- 2.Deisenhofer J, Michel H. Angew. Chem. Int. Ed. 1989;28:829–847. [Google Scholar]
- 3.Wald G. Angew. Chem. 1968;80:857–867. [Google Scholar]
- 4.Norrish RGW. Angew. Chem. 1968;80:868–881. [Google Scholar]
- 5.Kawata S, Kawata Y. Chem. Rev. 2000;100:1777–1788. doi: 10.1021/cr980073p. [DOI] [PubMed] [Google Scholar]
- 6.Hell SW. Angew. Chem. Int. Ed. 2015;54:8054–8066. doi: 10.1002/anie.201504181. [DOI] [PubMed] [Google Scholar]
- 7.Kathan M, Hecht S. Chem. Soc. Rev. 2017;46:5536–5550. doi: 10.1039/c7cs00112f. [DOI] [PubMed] [Google Scholar]
- 8.Dong M, Babalhavaeji A, Samanta S, Beharry AA, Woolley GA. Acc. Chem. Res. 2015;48:2662–2670. doi: 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- 9.Bleger D, Hecht S. Angew. Chem. Int. Ed. 2015;54:11338–11349. doi: 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- 10.Wyman GM. Chem. Rev. 1955;55:625–657. [Google Scholar]
- 11.Exelby R, Grinter R. Chem. Rev. 1965;65:247–260. [Google Scholar]
- 12.Bandara HM, Burdette SC. Chem. Soc. Rev. 2012;41:1809–1825. doi: 10.1039/c1cs15179g. [DOI] [PubMed] [Google Scholar]
- 13.Beharry AA, Woolley GA. Chem. Soc. Rev. 2011;40:4422–4437. doi: 10.1039/c1cs15023e. [DOI] [PubMed] [Google Scholar]
- 14.Irie M. Chem. Rev. 2000;100:1685–1716. doi: 10.1021/cr980069d. [DOI] [PubMed] [Google Scholar]
- 15.Berkovic G, Krongauz V, Weiss V. Chem. Rev. 2000;100:1741–1754. doi: 10.1021/cr9800715. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama Y. Chem. Rev. 2000;100:1717–1740. doi: 10.1021/cr980070c. [DOI] [PubMed] [Google Scholar]
- 17.Olson JP, Banghart MR, Sabatini BL, Ellis-Davies GCR. J. Am. Chem. Soc. 2013;135:15948–15954. doi: 10.1021/ja408225k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones G., II . In: Quantum Electronics–Principles and Applications. Duarte FJ, Hillman L, editors. Academic Press; San Diego: 1990. pp. 287–343. [Google Scholar]
- 19.Jing C, Cornish VW. Acc. Chem. Res. 2011;44:784–792. doi: 10.1021/ar200099f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J-R, Wong J-B, Kuo P-Y, Yang D-Y. Org. Lett. 2008;10:4823–4826. doi: 10.1021/ol8018902. [DOI] [PubMed] [Google Scholar]
- 21.Traven VF, Bochkov AY. Heterocycl. Commun. 2013;19:219–238. [Google Scholar]
- 22.Hara K, Sato T, Katoh R, Furube A, Ohga Y, Shinpo A, Suga S, Sayama K, Sugihara H, Arakawa H. J. Phys. Chem. B. 2003;107:597–606. [Google Scholar]
- 23.Anstaett P, Leonidova A, Janett E, Bochet CG, Gasser G. Chemphyschem. 2015;16:1863–1866. doi: 10.1002/cphc.201500178. [DOI] [PubMed] [Google Scholar]
- 24.Stranius K, Borjesson K. Sci. Rep. 2017;7:41145. doi: 10.1038/srep41145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velema WA, Szymanski W, Feringa BL. J. Am. Chem. Soc. 2012;136:2178–2191. doi: 10.1021/ja413063e. [DOI] [PubMed] [Google Scholar]
- 26.Tamai N, Miyasaka H. Chem. Rev. 2000;100:1875–1890. doi: 10.1021/cr9800816. [DOI] [PubMed] [Google Scholar]
- 27.Slavov C, Hartmann H, Wachtveitl J. Anal. Chem. 2015;87:2328–2336. doi: 10.1021/ac504348h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.