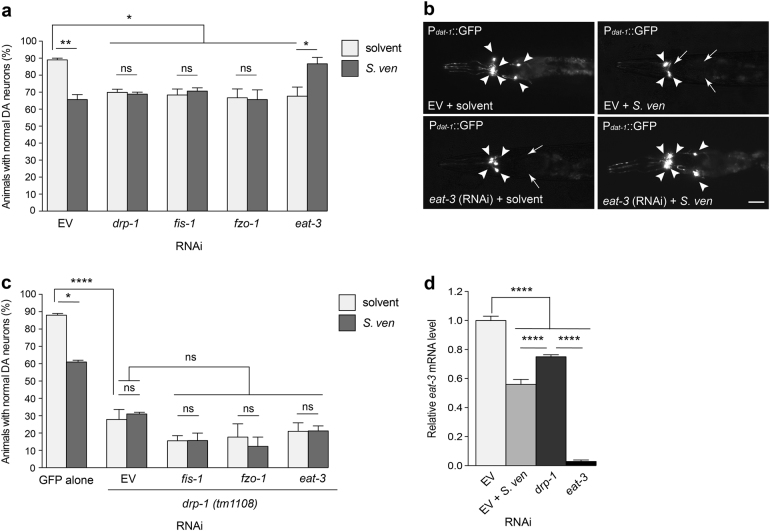
Fig. 4. Down-regulation of eat-3 suppresses neurotoxicity caused by the metabolite.
a, b RNAi knockdown of fission (drp-1 and fis-1) and fusion (fzo-1 and eat-3) components with solvent caused DA neurodegeneration at day 9 post-hatching. With metabolite exposure, RNAi treated with drp-1, fis-1, or fzo-1 animals did not exhibit enhanced DA neurotoxicity compared to the corresponding solvent control. In contrast, a combination of S. ven metabolite and RNAi targeting eat-3 resulted in significant resistance to neurotoxicity. The scale bar is 10 μm. These data are presented as mean ± S.E.M.; n = 30 animals per replicate, three independent replicates; one-way ANOVA with Tukey’s post hoc test for multiple comparisons. *P < 0.05, **P < 0.01. Representative images of the six anterior dopaminergic neurons are shown in (b), with degenerating or missing neurons marked by arrows and normal neurons indicated by arrowheads. c An RNAi-sensitive strain with GFP expression in DA neurons was crossed to drp-1(tm1108) mutant animals. Homozygosity was confirmed by PCR. Loss-of-function drp-1(tm1108) exacerbated DA neurotoxicity compared with GFP only solvent control at day 9 post-hatching. RNAi knockdown of eat-3 did not result in resistance of neurotoxicity as observed in Fig. 4a. Data are represented as mean ± S.E.M; n = 30 animals per replicate, three independent replicates; one-way ANOVA with Tukey’s post hoc test for multiple comparisons. *P < 0.05, *P < 0.0001. d eat-3 mRNA levels were measured by qRT-PCR in animals treated with solvent (EtAc), S. ven metabolite, drp-1 (RNAi), or eat-3 (RNAi). The animals treated with metabolite showed significant lower eat-3 mRNA levels than when drp-1 was depleted by RNAi. Data represented as mean ± S.E.M.; three replicates comprises at least 100 animals each; one-way ANOVA with Tukey’s post hoc test for multiple comparisons. ****P < 0.0001.