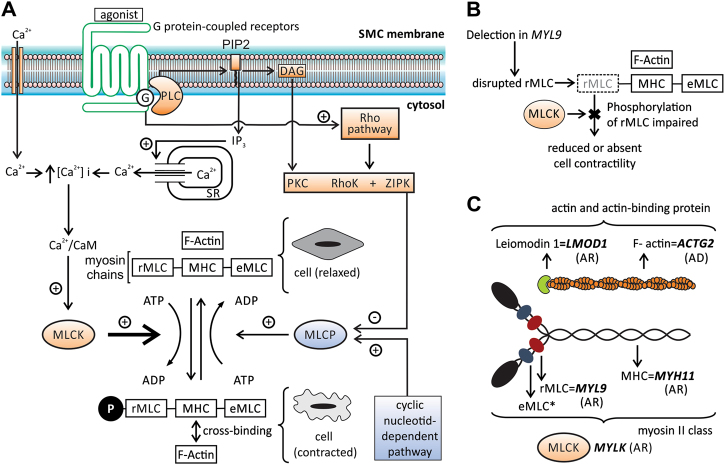
Fig. 3.
Contraction of smooth muscle cell: cellular pathway and the role of MYL9 and of other genes related to MMIHS. a Diagram illustrating some steps in the cellular pathway related to smooth muscle contraction. The phosphorylation of regulatory myosin light chain (rMLC) encoded by MYL9 is crucial to promote contraction and occurs at the end of this pathway (thick arrow). The increase in intracellular calcium concentration [Ca2+]i is an essential step to initiate the process, which is triggered by several factors (autonomic nervous system, hormones, local chemical, or mechanical stimulus). The [Ca2+]i increases due to two mechanisms: the influx of calcium ions (Ca2+) from extracellular to cytosol mediated by different channels (Ca2+ voltage-dependent and nonselective cation channels) and by the release of this ion from sarcoplasmatic reticulum (SR) through a G-protein- (G) mediated cascade. The binding of agonists to the G-protein-coupled receptor increases the phospholipase C (PLC) activity via G-protein leading to the production of inositol 1,4,5-triphophate (IP3) and diacylglycerol (DAG) from membrane phospholipids—phosphatidylinositol 4,5-biphosphate (PIP2). Protein G also activates the Rho pathway. IP3 binds to a specific receptor (IP3R) on SR causing the release of Ca2+ to the cytosol. The activation of the ryanodine receptor (RyR) on SR also contributes to the release of Ca2+ to the cytosol and both, IPR3 and RyR, are activated by increased [Ca2+]i (not shown). The intracellular Ca2+ (four molecules) binds to calmodulin (CaM) and this complex activates the myosin light chain kinase (MLCK). Activated MLCK phosphorylates the rMLC of myosin class II (also composed by myosin heavy chain—MHC and essential myosin light chain—eMLC), allowing actin–myosin binding and generating the force necessary to contract the cell. Dephosphorylation of rMLC occurs by the action of the myosin light chain phosphatase (MLCP), inducing the relaxation. MLCP is inactivated via phosphorylation mediated by kinases (PKC, RhoK, and ZIPK—activated by DAG and Rho G pathway) and activated by the cyclic nucleotide-dependent pathway. The increase in [Ca2+]i also stimulates the contraction by the pathway related to caldesmon, an actin-binding protein (not shown). b The presumed effect in smooth muscle contraction due to deletion in MYL9: the disruption of rMLC caused by deletion impairs the phosphorylation mediated by MLCK, leading to reduced or absent contraction. c The genes and the respective contractile filaments and inheritance patterns related to MMIHS—ACTG2 filamentous actin (F-actin), gamma-2 isoform, MYH11 myosin heavy chain (MHC) 11, MYLK myosin light chain kinase (MLCK), MYL9 regulatory myosin light chain (rMLC), LMOD1 leiomodin 1, an actin-binding protein. *eMLC encoded by MYL6 gene, not related to MMIHS until now, AD autosomal dominant inheritance, AR autosomal recessive inheritance. The information regarding smooth muscle contraction was based on different references [11, 28–30]