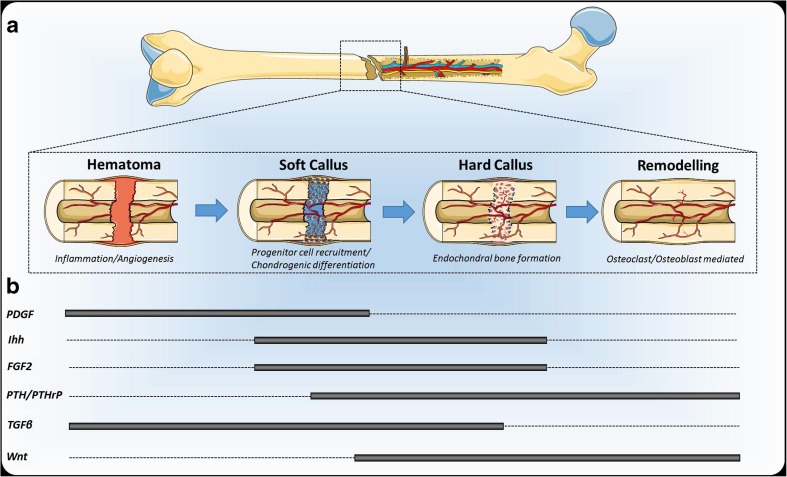
Fig. 1.
Main stages of long bone fracture repair and associated anabolic signaling pathways. a Long bone fractures generally heal through a process of endochondral ossification which progresses through a cartilaginous template. Stem cells are recruited from the periosteum and differentiate toward a hypertrophic chondrocyte phenotype; the matrix surrounding these cells subsequently serves as a scaffold for new bone formation. The process is completed through remodeling events controlled by osteoclasts and osteoblasts resulting in scar-free healing (created and adapted from Servier Medical Art). b Major anabolic signaling pathways which have recently been the focus of pharmaceutical targeting indicating their temporal contribution to the tissue formation processes. PDGF stimulates angiogenesis, macrophage recruitment/activation, and mesenchymal progenitor expansion [99]. Ihh plays a role in progenitor recruitment [90••], chondrocyte proliferation and PTHrP expression during endochondral ossification [100, 101]. FGF2 is a potent mitogen for mesenchymal progenitors as well as osteoprogenitors and chondroprogenitors [102, 103]. PTH can exert its function on several stages of fracture repair including cartilage formation, endochondral ossification, and remodeling [104], while PTHrP is known to control chondrocyte hypertrophy [100]. TGFβ signaling is involved in many stages of fracture repair, including the stimulation and proliferation of immune and mesenchymal cells, matrix synthesis, angiogenesis, and regulation of resorption (reviewed in [105]). Enhanced canonical Wnt signaling stimulates osteoprogenitor proliferation, chondrocyte hypertrophy, and decreases bone remodeling [104]