Figure 6.
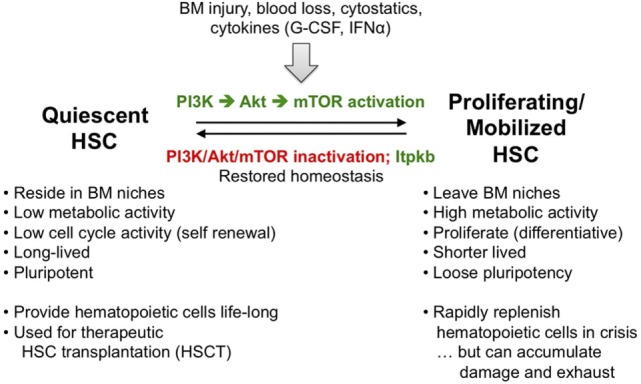
Hematopoietic stem cell (HSC) homeostasis is an exquisitely phosphoinositide 3-kinase (PI3K)-dependent process controlled by Itpkb. To ensure live-long hematopoiesis, HSC reside in BM niches and have low metabolic and cell cycle activity. As a consequence, HSC are long-lived and pluripotent. Stresses including BM injury, blood loss, exposure to cytostatic drugs or cytokines such as G-CSF or type 1 interferons activate and mobilize HSC to leave the BM niches, become metabolically active and proliferate. Some HSC daughter cells then differentiate into hematopoietic progenitors (Figure 2). As a consequence, activated HSC are short-lived and loose their pluripotency. This serves to rapidly replenish hematopoietic cells in a crisis or after HSC transplantation, but persistent HSC activation can lead to HSC damage and exhaustion, ultimately causing BM failure, anemia, immunodeficiencies, or blood cancer (85–87). To prevent this, resolution of HSC-activating stresses normally reverts them into quiescence once the activating stimuli subside. A key mediator of HSC activation that needs to be inactivated for re-entry into quiescence is PI3K signaling via Akt and downstream mammalian or mechanistic target of rapamycin (mTOR). In addition, we have identified Itpkb as a promoter of HSC quiescence and homeostasis that acts at least in part by inactivating Akt in HSC (26, 30).
