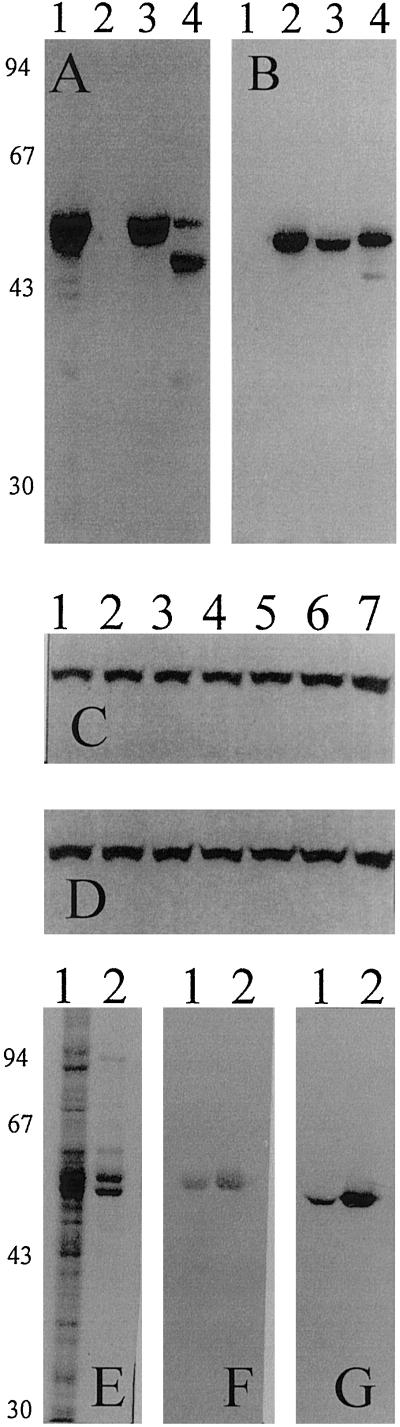
Figure 1.
Heterologous expression of the major barley endosperm AGPase in insect cells. A and B, Comparison of subunits of insect-cell-expressed and barley endosperm AGPases. Proteins from insect cell lysates containing only the expressed LS (lane 1, 10 μg), SS (lane 2, 10 μg), or both subunits (lane 3, 10 μg), and from barley endosperm extract (lane 4, 30 μg) were denatured, separated by 10% (w/v) SDS-PAGE, and transferred onto nylon membrane. Immunoblot was probed consecutively with antibodies recognizing the LS (A) and the SS (B). Molecular masses of the marker proteins are indicated on the left in kD. C and D, Stability at 4°C of expressed AGPase subunits in insect cell extract containing protease inhibitors. Insect cell extract containing the expressed AGPase (lane 1) was incubated at 4°C after extraction and aliquots were removed after 5 min (lane 2), 15 min (lane 3), 30 min (lane 4), 45 min (lane 5), 60 min (lane 6), and 120 min (lane 7), respectively. Proteins from different stages of incubation were denatured, separated on 10% (w/v) SDS-PAGE, and transferred onto nylon membrane that was then probed consecutively with antibodies recognizing the LS (C) and the SS (D). E to G, SDS-PAGE and immunoblots of the purified recombinant AGPase. Insect cell extract (lane 1, 20 μg) and purified recombinant AGPase (lane 2, 5 μg) were denatured, separated on 10% (w/v) SDS-PAGE, and either visualized with Coomassie Brilliant Blue R (E) or transferred onto nylon membrane that was then probed with antibodies recognizing the LS (F) and the SS (G). The molecular masses of marker proteins are indicated on the left in kD.