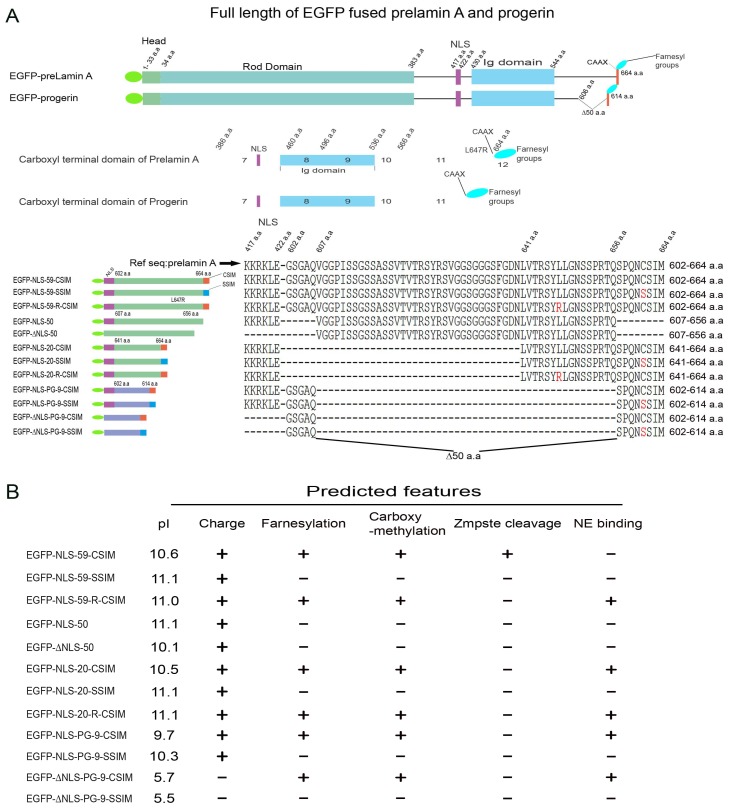
Figure 1.
Schematic representation of the C-terminal fragments of prelamin A and progerin. (A) Schematics depicting the structures of the EGFP–prelamin A and EGFP–progerin proteins, and constructs derived from their C-terminal domains. The C-terminal domain of the indicated protein is enlarged and the alignment of the construct sequences is shown. (1) Residues from 602 to 664 a.a. (amino acids) are marked as 59-CSIM/SSIM/R-CSIM, which represents the wild type, C661S mutant, and L647R mutant C-terminal fragments of prelamin A, respectively; (2) The 50 a.a. construct encodes the 50 a.a. in-frame deletion of prelamin A from 607 to 656 a.a.; (3) Residues from 641 to 664 a.a. of prelamin A are marked as 20-CSIM/SSIM/R-CSIM, which represent the short form of wild type, C661S mutant, and L647R mutant C-terminal fragments of prelamin A, respectively; (4) The PG-9-CSIM/SSIM, residues from 602–614 a.a. of progerin, represents the C-terminal fragment of progerin and its corresponding C661S mutant form. A nuclear localization signal (NLS, KKRKLE) was linked to a part of the constructs as indicated; (B) Based on the protein sequence properties, predicted features of all constructs were indicated: pI value, static charge, and post-translational properties, including farnesylation, carboxymethylation, Zmpste cleavage, and nuclear membrane binding ability.