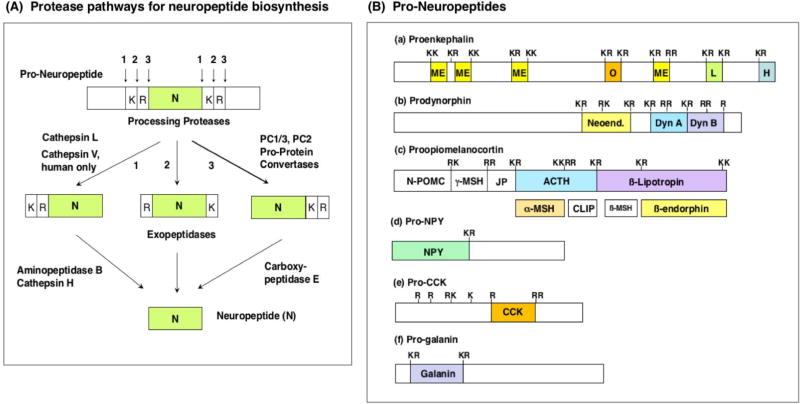
Figure 2. Protease Pathways to Convert Pro-Neuropeptides to Neuropeptides.
A. Protease pathways for neuropeptide biosynthesis. Pro-neuropeptides undergo proteolytic processing at dibasic residue cleavage sites by dual cysteine and serine subtilisin-like protease pathways. The cathepsin L cysteine protease cleavages pro-neuropeptides at paired basic residues. Resultant peptide intermediates require removal of N-terminal basic residues by aminopeptidase B or cathepsin H, and removal of C-terminal basic residues by carboxypeptidase E (CPE). The human-specific cathepsin V cysteine protease functions only in human cells for neuropeptide production, because the cathepsin V gene is present only in the human genome (and not in the genome of other species of organisms). The serine protease pathway consists of the pro-protein convertases (PC) PC1/3 and PC2 that cleave at paired basic residues. Resultant peptide intermediates then require removal of C-terminal basic residues by CPE.
B. Pro-neuropeptide structures. Schematic illustration of the opioid pro-neuropeptide precursors are shown for proenkephalin (PENK), prodynorphin (PDYN), and proopiomelanocortin (POMC), as well as the precursors of neuropeptide Y (NPY), cholecystokinin (CCK), and galanin (GAL). Mature neuropeptides are indicated in colored areas within the pro-neuropeptides. Neuropeptides within the precursors are typically flanked by paired basic residues (Lys-Arg (KR), Lys-Lys(KK), Arg-Lys (RK), and Arg-Arg (RR)), and occasionally at monobasic Arg sites, that are recognized and cleaved by processing proteases to generate mature neuropeptides.