Figure 2.
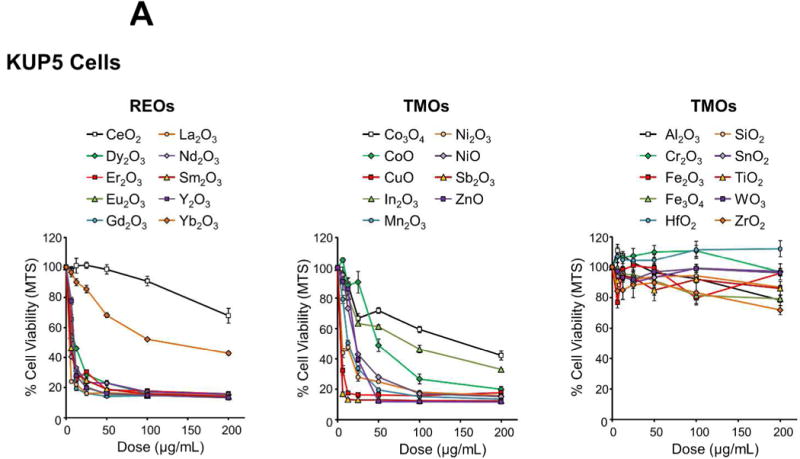
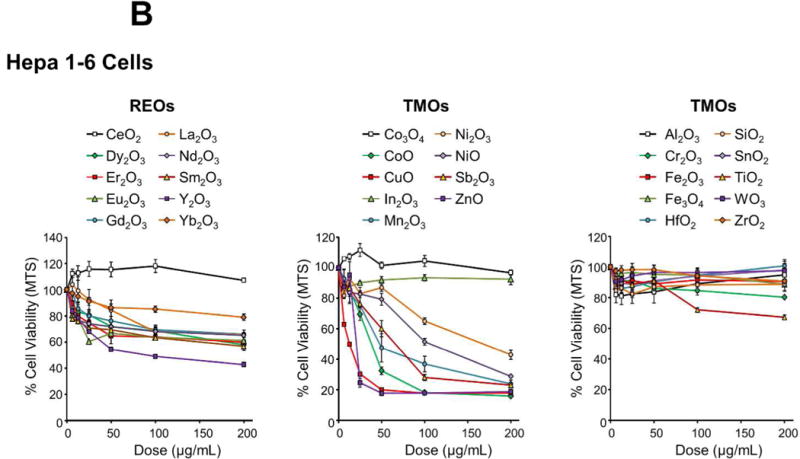
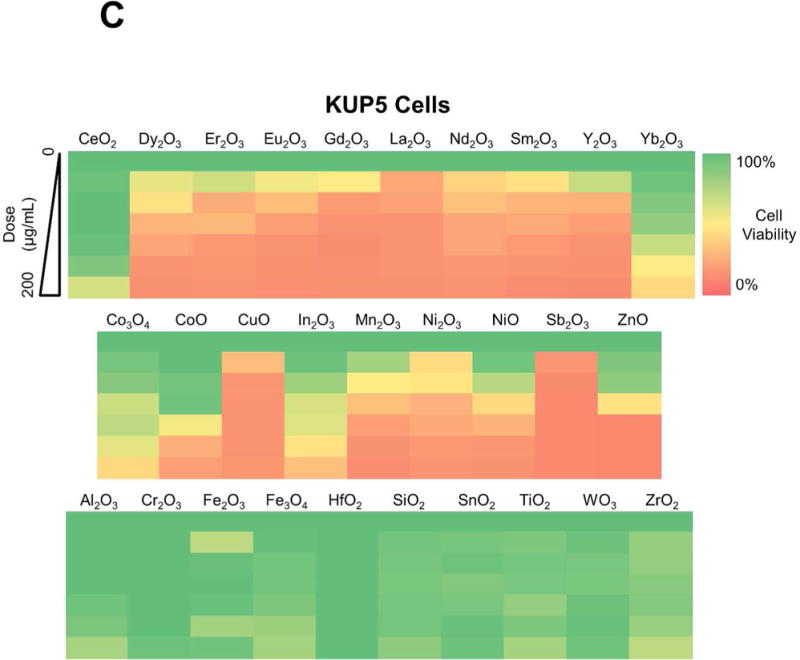
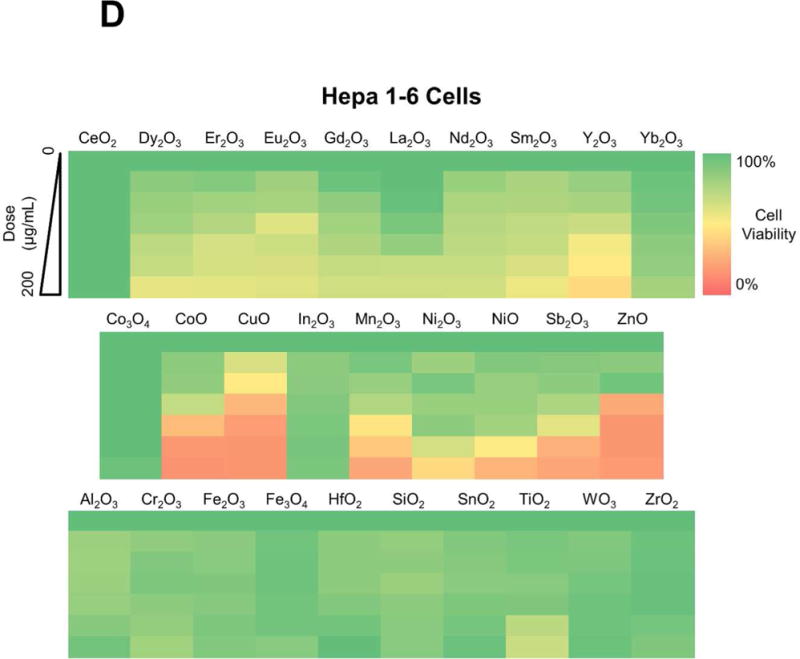
Cytotoxicity screening of MOx nanoparticles in KUP5 and Hepa 1-6 cells. Use of an MTS assay to assess the viability of (A) KUP5 and (B) Hepa 1-6 cells after exposure to REO and TMO nanoparticles for 24 h over a dose range of 6.25-200 μg/mL. The results are reported in 3 material categories, namely for REOs, redox-active TMOs and inert TMOs. The viability of non-treated control cells was regarded as 100%. The results were also expressed as heat maps for (C) KUP5 and (D) Hepa 1-6 cells, calibrated against the color scale in the sidebar.
