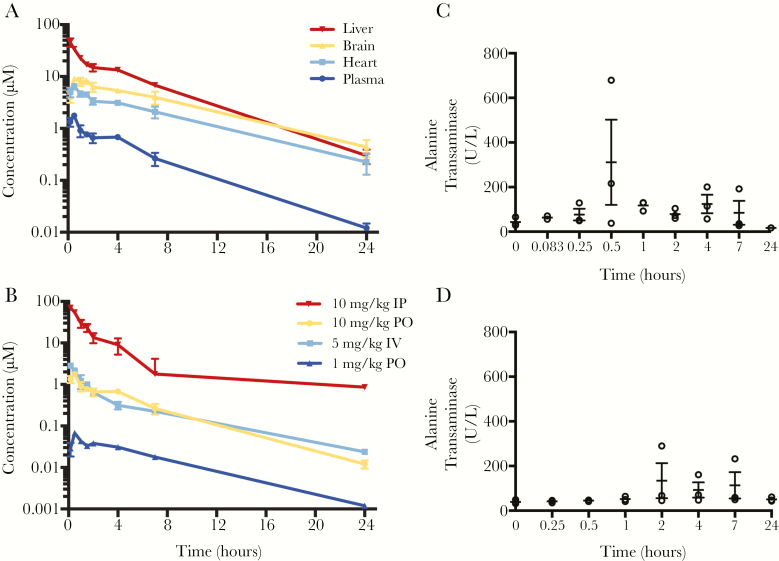
Figure 2.
Pharmacokinetic profile of compound 3. A, Tissue-specific distribution of compound 3 following 10 mg/kg oral (PO) administration over 24 hours. B, Establishing linearity of exposure between 10 mg/kg and 1 mg/kg doses in plasma. Intravenous (IV) administration was used to calculate bioavailability (F) and volume of distribution (Vdss). Plasma levels of alanine transaminase (ALT; C) were determined at several time points over the treatment window following 10 mg/kg intraperitoneal (IP) administration as an indicator of drug-related hepatotoxicity; T0 = pretreatment. D, Vehicle-only control showing spotty increases in alanine transaminase unrelated to administration of the compound.