Abstract
Objective
To identify and describe 2‐year trajectories of fear‐avoidance beliefs on physical activity and to identify predictors of these trajectories in people with rheumatoid arthritis (RA).
Methods
We included 2,569 persons with RA (77% women, mean age 58 years). Data on fear‐avoidance beliefs (Fear‐Avoidance Beliefs Questionnaire physical activity subscale [FABQ‐PA]; range 0–24), sociodemographics, disease‐related variables, self‐efficacy, and health‐enhancing physical activity (HEPA) were collected from registers and by questionnaires at baseline, 14, and 26 months. K‐means cluster analysis was used to identify fear‐avoidance trajectories, and multinomial logistic regression was used to identify predictors of trajectory membership.
Results
Three trajectories of fear‐avoidance beliefs were identified: low (n = 1,060, mean FABQ‐PA = 3), moderate (n = 1,043, mean FABQ‐PA = 9), and high (n = 466, mean FABQ‐PA = 15). Consistent predictors of being in the high fear‐avoidance trajectory versus the other 2 trajectories were high activity limitation, male sex, income below average, not performing current HEPA, and elevated anxiety/depression. In addition, less consistent predictors such as shorter education, more pain, and low exercise self‐efficacy were also identified.
Conclusion
Stable trajectories of fear‐avoidance beliefs on physical activity exist among people with RA. Fear‐avoidance may be targeted more effectively by tailoring physical activity promotion to vulnerable socioeconomic groups, men, and those with high activity limitation and anxiety/depression.
Introduction
Pain, fatigue, and depression 1 in many cases lead to activity limitation 2 and are common among persons with rheumatoid arthritis (RA). Better use of disease‐modifying drugs, including biologics, during the past decades has improved inflammation control and the course of disease 1, 3, but many persons under treatment still report pain and fatigue 1. Persons with RA also experience an increased risk of cardiovascular comorbidity, partly due to the disease‐specific inflammatory processes 4.
Significance & Innovations.
Distinct 2‐year trajectories of fear‐avoidance beliefs on physical activity can be identified in persons with rheumatoid arthritis.
Stable, high fear‐avoidance is predicted by high activity limitation, male sex, income below average, not performing health‐enhancing physical activity, and elevated levels of anxiety/depression.
Fear‐avoidance may be targeted more effectively by tailoring physical activity promotion to vulnerable socioeconomic groups, men, and those with high activity limitation and anxiety/depression.
To address disease symptoms and the increased risk of cardiovascular comorbidity, sufficient physical activity, including aerobic exercise and muscle‐strength training, is recommended 5. Health‐enhancing physical activity (HEPA) is generally defined as a minimum of 150 weekly minutes of moderate, or 75 weekly minutes of intense, physical activity with the addition of muscle strength training twice a week 6. The HEPA recommendations are similar for populations with and without long‐term health conditions such as RA 6.
Despite the beneficial effects of HEPA, many persons with RA do not reach or maintain the recommended HEPA levels 7, 8. To explore and potentially influence physical activity behavior, numerous correlates have been identified in the general population 9 and in RA 10. For people with RA, psychological and contextual variables seem to be stronger correlates of physical activity than disease‐related and sociodemographic ones 8, 10, 11. Self‐efficacy, defined as a person's confidence in performing a specific behavior 12, and motivation for being physically active are 2 important psychological determinants 8, 10, 11.
Fear‐avoidance belief about pain and its consequences is one psychological variable found to predict long‐term disability 13 and to moderate treatment effects 14 in subacute nonspecific back pain. A theoretical model based on clinical observations of patients with persistent pain describes the role of fear‐avoidance as part of a vicious cycle of psychological and behavioral events. Negative appraisal of pain due to negative affectivity and/or threatening illness information may lead to catastrophic cognitions about the worst possible outcome. This negative appraisal leads to fear, avoidant behaviors, and hypervigilance to bodily sensations, followed by disuse, depression, and disability, which in turn maintain the pain experience, fear, and avoidance 15, 16. Intervention studies indicate that it is possible to affect fear‐avoidance by use of graded exposure treatment in patients with long‐term back pain 17, 18, 19 and complex regional pain syndromes 20. Such treatment includes patient education and systematic exposure to feared activities with gradually increasing difficulty, tailored to each patient 20. Progressive, person‐centered resistance exercise has also proven effective in reducing fear‐avoidance beliefs in women with fibromyalgia 21. However, if not addressed with appropriate treatment, fear‐avoidance beliefs seem persistent in patients with chronic low back pain, while they are spontaneously reduced over time in patients with acute back pain 22.
In RA, the role of fear‐avoidance and its development over time have been minimally explored and the few existing studies are inconclusive, reporting positive correlations with pain intensity 23, 24 but not with physical activity 24, 25. RA is a long‐term condition, characterized by unpredictable flares and remissions with subsequent variations in pain, that also affects many other physical, psychological, and contextual aspects of health and functioning. Thus, a wide approach based on the fear‐avoidance model and longitudinal data would benefit a better understanding of the role of fear‐avoidance beliefs and their development over time in people with RA.
The development of fear‐avoidance beliefs may be described by presenting changes in mean levels over time in a total sample. However, such analyses do not take into account that several different patterns may coexist within that sample, and consequently a major part of the potential heterogeneity in fear‐avoidance may not be identified or explained. An alternative method is to identify a number of clinically relevant patterns using longitudinal cluster analysis. Such analyses identify groups of individuals, defined by a specific pattern, or trajectory, over time 26. To our knowledge, no previous study has investigated trajectories of fear‐avoidance beliefs over time in a large, well‐defined sample of people with RA. The aim of the present study was to identify and describe groups displaying different trajectories of fear‐avoidance beliefs over 2 years and to identify biopsychosocial, theory‐based baseline predictors for each trajectory in a large cohort of people with RA.
Subjects And Methods
Design
This longitudinal study used data from the Swedish Rheumatology Quality registers, which at the time of data collection (2010) included approximately 27,000 individuals with RA. Six rheumatology clinics were selected to represent university hospitals (n = 2) and county hospitals (n = 4) in different parts of Sweden.
Participants
The 6 participating clinics had 9,560 registered patients diagnosed with RA according to the 1987 criteria from the American College of Rheumatology 27. Inclusion criteria were ages ≤75 years and a Stanford Health Assessment Questionnaire disability index (HAQ DI) of ≤2.0. After additional exclusion of registered patients who had died (n = 164), emigrated (n = 24), or had protected identity (n = 14), 5,391 eligible individuals were mailed a questionnaire. Of these, 3,152 answered and were mailed the same questionnaire again 14 and 26 months later. The present study sample includes the 2,569 individuals completing the questionnaire at least twice: at baseline and at 14 and/or 26 months.
Measures
Dependent variable
Fear‐avoidance beliefs were assessed with a subscale from the Fear‐Avoidance Beliefs Questionnaire (FABQ), consisting of the following 4 items on beliefs about physical activity causing pain and injury: pain is caused by physical activity, physical activity makes one's pain worse, physical activity might be harmful, and one should not do physical activity that might worsen one's pain. These 4 items constitute the physical activity subscale (FABQ‐PA), identified through principal component analysis of the original 16‐item FABQ 28. Ratings are given on 0–6 scales, where 0 = do not agree at all and 6 = agree completely, with a sum score of 0–24. Values over 15 on FABQ‐PA have been suggested to indicate high fear‐avoidance 29 and cutoffs between 14 and 16 have been used to dichotomize samples in previous studies 30, 31. FABQ has demonstrated good test–retest reliability and internal consistency in patients with acute low‐back pain 32.
Independent variables
Sociodemographic data on sex and age were retrieved from the Swedish Rheumatology Quality registers. Data on education, income, number of children and adults in the household, and all additional data were collected by postal questionnaires.
Disease‐related data on comorbidity were assessed by number and name of additional diagnoses besides RA. Pain, fatigue, and general health perception were rated on visual analog scales (range 0–100), where 100 indicated the worst condition. For the analyses, pain was categorized as 0–29, 30–54, and 55–100 according to Collins et al 33, while fatigue and general health were divided into tertiles based on the distribution of the present sample. Activity limitation was assessed by the HAQ DI, which includes 20 items. Eight activities of daily living (e.g., dressing, eating, and performing hygiene) were rated on a 0–3 scale, where 0 = without difficulty and 3 = unable to do. A total HAQ DI score was created by calculating the mean of the 20 items. The score was categorized into 3 groups for analysis (0, 0.1–1.0, or >1.0) as HAQ 1.0 and above indicates difficulties in every activity of daily living 34. Anxiety and depression were assessed by 1 item from the EuroQoL 5‐domain questionnaire (EQ‐5D) 35 where 1 = “I am not anxious or depressed,” 2 = “I am moderately anxious or depressed,” and 3 = “I am extremely anxious or depressed.” EQ‐5D has demonstrated satisfactory concurrent validity by high correlations with disability measures in RA 36.
Self‐efficacy for exercise was assessed by the Exercise Self‐Efficacy Scale (ESES) using the stem “How confident are you to exercise…” followed by 6 items describing common barriers for exercise, e.g., when physically fatigued. Ratings are given on a 1–6 scale, where 1 = not at all confident and 6 = very confident, adding up to a total score with a range of 6–36. The ESES is reported to have sufficient internal consistency and concurrent validity 37. Tertiles based on the present sample distribution were created for analysis.
Current HEPA was assessed using the short version of the International Physical Activity Questionnaire (IPAQ). This questionnaire includes items on physical activity at 3 intensity levels (vigorous, moderate, and walking) and 4 domains (work, home, transportation, and leisure time). The short version has acceptable test–retest reliability and criterion‐related validity compared to accelerometers 38. Minutes of vigorous, moderate, and walking intensity activity per week were summed and converted to hours per week for each individual. Based on the IPAQ data, we created the dichotomous variable Current HEPA, indicating whether the recommendations on total moderately/vigorously intense physical activity (150 minutes of moderate intensity or 75 minutes of vigorous intensity plus 2 muscle‐strength training sessions) during the past week were met or not. Maintained HEPA was assessed using the Exercise Stage Assessment Instrument (ESAI) 39, which was modified from 1–2 items to suit the present study. The first item defines performance of aerobic physical activity as that of moderate intensity during a minimum of 30 minutes at least 5 times per week. The second item defines performance of muscle strength training as resistance training at least twice a week. Both items are followed by the question: “Are you physically active according to this description?” Five response options are given, based on the stages in the Transtheoretical Model 40: “No, and I don't intend to be within the next six months,” “No, but I intend to be within the next six months,” “No, but I intend to be within the next 30 days,” “Yes, and I have been for less than six months,” and “Yes, I have been for six months or longer.” To be coded as maintained HEPA, the last response option was required for both items. All other combinations were coded as not maintained HEPA. The original ESAI has demonstrated sufficient test–retest reliability 41 and construct validity with other exercise stage‐of‐change measures 42.
Statistical analysis
Descriptive data were calculated for the whole sample at baseline using means and SDs or percentages, as appropriate. K‐means cluster analysis for longitudinal data, using the KmL 26 package in R 43, was used to identify distinct trajectories of fear‐avoidance beliefs, which were measured 3 times (at baseline, 14‐ and 26‐month followup). In brief, the K‐means method for cluster analysis begins by randomly assigning each individual participant's trajectory of fear‐avoidance beliefs into one of the K groups. A model is fit to each group to create 1 trajectory to represent each of the K groups. The distance between each individual trajectory and group trajectory is calculated, and participants are moved to the closest group. This algorithm is then repeated until all individual participant trajectories stop moving. As cluster analysis requires a priori specification of the number of clusters (K), the analysis was run 4 times with a priori numbers of clusters from 2 to 5. To assess model fit, the Calinski‐Harabasz criterion, which is a ratio of between‐cluster means and within‐cluster covariance, along with consideration of clinical relevance, was used to select the number of clusters that best fit the data.
Multinomial logistic regression was then used to calculate odds ratios and 95% confidence intervals for the association between potential predictors of trajectory membership and each trajectory, with trajectory as the outcome. Correlations among potential predictors were examined to assure absence of collinearity.
Missing data were <5% in all variables except for self‐efficacy for exercise, which had 10% missing. Multiple imputation was used to impute missing data in potential predictors for the multinomial logistic regression, which included sociodemographic, disease‐related, self‐efficacy, and physical activity variables. Analyses were performed with and without imputed data. As similar results were seen in imputed versus nonimputed analyses, we present the results using the imputed data.
The study was carried out in compliance with the Helsinki Declaration. Ethical approval was obtained from the Stockholm Regional Ethical Review Board (2010/1232‐31/1 and 2011/1241‐32). Participants were invited to the study in an information letter, and they consented by returning their completed questionnaires.
Results
Three distinct, stable trajectories of fear‐avoidance beliefs deemed to be of clinical relevance were identified. These trajectories represented a low group (n = 1,060, 41%) with average FABQ‐PA of 3, a moderate group (n = 1,043, 41%) with average FABQ‐PA of 9, and a high group (n = 466, 18%), with average FABQ‐PA of 15 (Figure 1). Baseline descriptive data for the total sample (n = 2,569) and separately by the 3 trajectories are shown in Table 1, demonstrating differences between groups in all included potential predictors except number of adults in household (P = 0.362).
Figure 1.
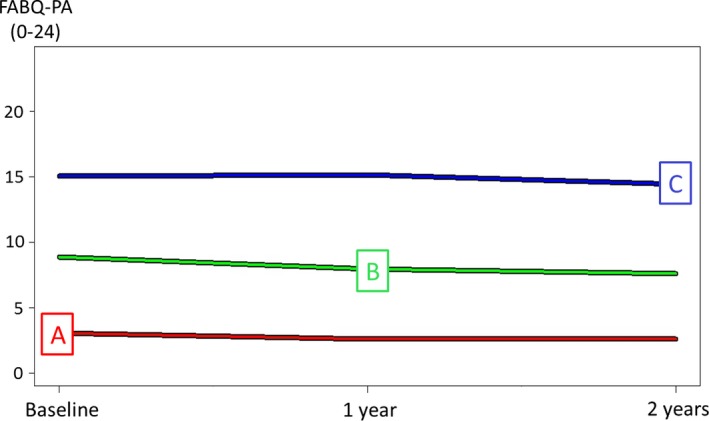
Three trajectories of fear‐avoidance beliefs identified by K‐means cluster analysis: A = low (41% of the total sample), B = moderate (41% of the total sample), and C = high (18% of the total sample). Total sample n = 2,569. Values indicate the average over a 2‐year period. FABQ‐PA = Fear‐Avoidance Beliefs Questionnaire physical activity subscale.
Table 1.
Baseline characteristics for the total study sample, low (n = 1,060), moderate (n = 1,043), and high (n = 466) fear‐avoidance groupsa
| Total | Low | Moderate | High | P | |
|---|---|---|---|---|---|
| Sex, no. | 2,569 | 1,060 | 1,043 | 466 | – |
| Women | 1875 (73) | 817 (77) | 761 (73) | 297 (64) | < 0.0001 |
| Men | 694 (27) | 243 (23) | 282 (27) | 169 (36) | – |
| Age, no. (mean ± SD) | 2,569 (60 ± 11) | 1,060 (58 ± 12) | 1,043 (59 ± 10.8) | 466 (62 ± 9) | < 0.0001 |
| 18–34 | 90 (4) | 51 (5) | 35 (3) | 4 (1) | < 0.0001 |
| 35–54 | 624 (24) | 285 (27) | 250 (24) | 89 (19) | – |
| ≥55 | 1,873 (73) | 724 (68) | 758 (73) | 373 (80) | – |
| Education, no. | 1,746 | 1,054 | 1,035 | 457 | – |
| University | 888 (50) | 473 (45) | 321 (31) | 94 (21) | < 0.0001 |
| College | 672 (15) | 252 (24) | 279 (27) | 141 (31) | – |
| Other | 300 (17) | 110 (10) | 147 (14) | 43 (9) | – |
| Basic | 686 (39) | 219 (21) | 288 (28) | 179 (39) | – |
| Swedish average income, no. | 2,510 | 1,044 | 1,017 | 449 | – |
| Below | 1,256 (50) | 403 (39) | 530 (52) | 323 (72) | < 0.0001 |
| Above | 1,254 (50) | 641 (61) | 487 (48) | 126 (28) | – |
| Ages <18 years, no. | 2,550 | 1,053 | 1,037 | 460 | – |
| 0 | 2,138 (84) | 855 (81) | 885 (85) | 398 (87) | 0.002 |
| 1 | 189 (7) | 82 (8) | 76 (7) | 31 (7) | – |
| ≥2 | 223 (9) | 116 (11) | 76 (7) | 31 (7) | – |
| Additional adults in household, no. | 2,498 | 1,054 | 1,033 | 456 | – |
| 0 | 578 (23) | 232 (22) | 230 (22) | 116 (25) | 0.362 |
| 1 | 1,620 (65) | 694 (66) | 681 (66) | 290 (64) | – |
| 2–3 | 276 (11) | 118 (11) | 112 (11) | 46 (10) | – |
| >3 | 24 (1) | 10 (1) | 10 (1) | 4 (1) | – |
| Comorbidities, no.b | 2,569 | 1,060 | 1,043 | 466 | – |
| 0 | 1,139 (44) | 561 (53) | 426 (41) | 152 (33) | < 0.0001 |
| 1 | 437 (17) | 176 (17) | 185 (18) | 76 (16) | – |
| ≥2 | 993 (39) | 323 (30) | 432 (41) | 238 (51) | – |
| Pain, no. (mean ± SD)c | 2,563 (32 ± 25) | 1,059 (21 ± 21) | 1,039 (36 ± 24) | 465 (48 ± 25) | < 0.0001 |
| Low (0–29) | 1,389 (54) | 790 (75) | 469 (45) | 130 (28) | < 0.0001 |
| Moderate (30–54) | 594 (23) | 167 (16) | 297 (29) | 130 (28) | – |
| High (>55) | 580 (23) | 102 (10) | 273 (26) | 205 (44) | – |
| Fatigue, no. (mean ± SD)c | 2,149 (39 ± 27) | 1,059 (28 ± 24) | 1,037 (43 ± 25) | 465 (53 ± 25) | < 0.0001 |
| Low, lowest tertile | 890 (41) | 532 (50) | 283 (27) | 75 (16) | < 0.0001 |
| Moderate, middle tertile | 861 (40) | 341 (32) | 373 (36) | 147 (32) | – |
| High, highest tertile | 810 (38) | 186 (18) | 381 (37) | 243 (52) | – |
| Health, no. (mean ± SD)c | 2,513 (34 ± 25) | 1,041 (23 ± 23) | 1,019 (37 ± 23) | 453 (50 ± 24) | < 0.0001 |
| Good, lowest tertile | 885 (35) | 558 (54) | 269 (26) | 58 (13) | < 0.0001 |
| Moderate, middle tertile | 852 (34) | 315 (30) | 381 (37) | 129 (28) | – |
| Poor, highest tertile | 803 (32) | 168 (16) | 369 (36) | 266 (59) | – |
| Activity limitation, no. (mean ± SD)d | 2,556 (0.62 ± 0.6) | 1,054 (0.35 ± 0.4) | 1,043 (0.70 ± 0.6) | 462 (1.01 ± 0.6) | < 0.0001 |
| Low, 0 | 649 (25) | 437 (41) | 178 (17) | 34 (7) | < 0.0001 |
| Moderate, 0.1–1.0 | 1,348 (53) | 528 (50) | 592 (57) | 228 (49) | – |
| High, 1.1–3.0 | 562 (22) | 89 (8) | 273 (26) | 200 (43) | – |
| Anxiety/depression, no.e | 2,558 | 1,055 | 1,040 | 463 | – |
| Low, no anxiety/depression | 1,677 (66) | 807 (76) | 651 (63) | 219 (47) | < 0.0001 |
| Moderate, to some extent | 835 (33) | 241 (23) | 372 (36) | 222 (48) | – |
| High, extremely | 46 (2) | 7 (1) | 17 (2) | 22 (5) | – |
| Self‐efficacy for exercise, no. (mean ± SD)f | 2,303 (31 ± 13) | 968 (35 ± 13) | 940 (29 ± 12) | 395 (27 ± 13) | < 0.0001 |
| Low, lowest tertile | 760 (33) | 251 (26) | 336 (36) | 173 (44) | < 0.0001 |
| Moderate, middle tertile | 733 (32) | 272 (28) | 332 (35) | 129 (33) | – |
| High, highest tertile | 810 (35) | 445 (46) | 272 (29) | 93 (24) | – |
| Current HEPA, no.g | 2,560 | 1,056 | 1,039 | 465 | – |
| Yes, >150 minutes | 1,829 (71) | 855 (81) | 718 (69) | 256 (55) | < 0.0001 |
| No, <150 minutes | 731 (29) | 201 (19) | 321 (31) | 209 (45) | – |
| Maintained HEPA, no.h | 2,432 | 1,010 | 987 | 435 | – |
| Yes, >6 months | 284 (12) | 166 (16) | 88 (9) | 30 (7) | < 0.0001 |
| No, <6 months | 2,148 (88) | 844 (84) | 899 (91) | 405 (93) | – |
Values are the number (%) unless indicated otherwise. Comparisons between all 3 groups are based on chi square test or unpaired t‐test. Where numbers do not add up to the number of total group size (2,769, 1,060, 1,043, 466), there are missing data. VAS = visual analog scale; HAQ DI = Health Assessment Questionnaire disability index; EQ‐5D = EuroQoL 5‐domain questionnaire; ESES = Exercise Self‐Efficacy Scale; HEPA = health‐enhancing physical activity; IPAQ = International Physical Activity Questionnaire; ESAI = Exercise Stage Assessment Instrument.
Predominantly cardiovascular disease, lung disease, and additional musculoskeletal conditions.
Measured by VAS, range 0–100.
Measured by HAQ DI, range 0–3.
Measured by EQ‐5D.
Measured by ESES, range 6–60.
Measured by IPAQ.
Measured by ESAI.
The results are presented by contrasting the high group versus the moderate and the low fear‐avoidance groups. Consistent predictors (i.e., demonstrating high odds ratio values and distinguishing the trajectory with high fear‐avoidance from the other 2 trajectories) of being in the high group were high activity limitation, male sex, income below average, not performing HEPA, and anxiety/depression. Being in the high versus the low group was predicted by education below university level, high pain intensity, moderate anxiety/depression, and low self‐efficacy for exercise. The results for all potential predictors of being in the high versus the other 2 groups are shown in Table 2.
Table 2.
Baseline predictors of being in the high (n = 466) fear‐avoidance trajectory versus the low (n = 1,060) and the moderate (n = 1,043) trajectories based on multinomial logistic regression analysisa
| High vs low | High vs moderate | |||
|---|---|---|---|---|
| Baseline predictor | OR (95% CI) | P | OR (95% CI) | P |
| Women vs. men | 0.30 (0.22–0.41) | < 0.0001 | 0.51 (0.39–0.67) | < 0.0001 |
| Ages 35–54 vs. 18–34, years | 3.17 (1.01–9.96) | 0.049 | 3.08 (1.01–9.37) | 0.048 |
| Ages >55 vs. 18–34, years | 3.01 (0.95–9.47) | 0.06 | 4.04 (1.32–12.35) | 0.015 |
| Education | ||||
| High school vs. university | 1.84 (1.29–2.62) | 0.001 | 1.33 (0.96–1.85) | 0.089 |
| Other vs. university | 1.11 (0.68–1.80) | 0.672 | 0.71 (0.46–1.11) | 0.137 |
| Basic vs. university | 1.99 (1.38–2.86) | 0.0002 | 1.34 (0.96–1.87) | 0.085 |
| Income above vs. below Swedish average | 0.42 (0.31–0.57) | < 0.0001 | 0.50 (0.38–0.65) | < 0.0001 |
| Children <18 years 1 vs. 0 | 1.71 (0.99–2.98) | 0.056 | 1.52 (0.92–2.51) | 0.102 |
| Children <18 years 2+ vs. 0 | 1.53 (0.86–2.71) | 0.146 | 1.89 (1.09–3.25) | 0.023 |
| Comorbidities 1 vs. 0 | 1.31 (0.90–1.90) | 0.160 | 1.04 (0.74–1.48) | 0.804 |
| Comorbidities ≥2 vs. 0 | 1.45 (1.08–1.95) | 0.014 | 1.12 (0.86–1.47) | 0.399 |
| Pain VAS, moderate vs. low | 1.65 (1.10–2.46) | 0.015 | 0.93 (0.64–1.35) | 0.707 |
| Pain VAS, high vs. low | 2.58 (1.58–4.22) | 0.0001 | 1.22 (0.79–1.87) | 0.370 |
| Fatigue VAS, moderate vs. low | 1.25 (0.85–1.85) | 0.256 | 1.04 (0.71–1.52) | 0.831 |
| Fatigue VAS, high vs. low | 1.47 (0.94–2.31) | 0.094 | 1.09 (0.71–1.67) | 0.687 |
| Health VAS, moderate vs. poor | 1.34 (0.88–2.05) | 0.176 | 1.10 (0.72–1.68) | 0.644 |
| Health VAS, good vs. poor | 1.79 (1.03–3.10) | 0.04 | 1.64 (0.98–2.73) | 0.06 |
| Activity limitation (HAQ DI) | ||||
| Moderate vs. low | 3.10 (2.01–4.78) | < 0.0001 | 1.60 (1.04–2.48) | 0.033 |
| High vs. low | 7.14 (4.19–12.19) | < 0.0001 | 2.11 (1.28–3.47) | 0.003 |
| Anxiety/depression (EQ‐5D) | ||||
| Moderate vs. low | 1.50 (1.13–2.01) | 0.006 | 1.31 (1.01–1.69) | 0.039 |
| High vs. low | 3.56 (1.33–9.56) | 0.012 | 2.68 (1.31–5.49) | 0.007 |
| Self‐efficacy for exercise (ESES) | ||||
| Moderate vs. low | 0.87 (0.63–1.22) | 0.422 | 0.90 (0.67–1.20) | 0.458 |
| High vs. low | 0.53 (0.38–0.76) | 0.001 | 0.87 (0.63–1.19) | 0.373 |
| Current HEPA (IPAQ), yes vs. no | 0.54 (0.41–0.72) | < 0.0001 | 0.70 (0.55–0.89) | 0.004 |
| Maintained HEPA (ESAI), yes vs. no | 0.60 (0.37–0.96) | 0.033 | 0.88 (0.55–1.40) | 0.597 |
OR = odds ratio; 95% CI = 95% confidence interval; VAS = visual analog scale; HAQ DI = Health Assessment Questionnaire disability index; EQ‐5D = EuroQoL 5‐domain questionnaire; ESES = Exercise Self‐Efficacy Scale; HEPA = health‐enhancing physical activity; IPAQ = International Physical Activity Questionnaire; ESAI = Exercise Stage Assessment Instrument.
Discussion
To our knowledge, this is the first study using longitudinal data to describe trajectories of fear‐avoidance beliefs on physical activity in a large well‐defined sample of people with RA. Three stable trajectories with low, moderate, and high levels of fear avoidance beliefs were identified. The most consistent predictors of high fear‐avoidance were high activity limitation, male sex, income below average, not currently performing HEPA, and elevated levels of anxiety/depression.
The smallest trajectory group was the one with high fear‐avoidance beliefs, indicating that most people with RA manage their disease without developing high levels of fear‐avoidance. Cross‐sectional analyses of baseline data in this same cohort indicated similar results 23, reporting a median of 7 on FABQ‐PA (range 0–24). The results also align with research on patients with nonspecific musculoskeletal pain, demonstrating that the majority recover and return to activity and work, while approximately 10–15% of patients with acute low‐back pain develop a persistent disabling condition 44, mediated mainly by psychological variables such as fear‐avoidance beliefs 45.
The stability of trajectories in the present study is in line with a previous 3‐year longitudinal study on patients with nonspecific low‐back pain 46, indicating that once fear‐avoidance beliefs have been established, they are not likely to dissolve automatically. For health professionals, it is thus important not only to assess physical function, but also to identify and challenge unhelpful negative cognitions about physical activity as potentially causing pain and injury. Since there is evidence for graded exposure treatment to reduce fear‐avoidance in long‐term back pain 17, 18, 19, future studies are motivated to evaluate its feasibility and outcome in patients with RA. In addition, the effectiveness of person‐centered, progressive resistance exercise to reduce fear‐avoidance beliefs in patients with fibromyalgia 21 may be applicable in RA as well.
High fear‐avoidance beliefs were predicted by a number of variables included in the theoretical fear‐avoidance model developed by Lethem et al 15 and Vlaeyen and Linton 16. The model describes a vicious circle where each variable influences, but is also influenced by, other variables in a circular chain. Among those, the strongest predictor in our study was activity limitation. The correlation between activity limitation and fear‐avoidance beliefs has been confirmed in a recent review of studies on patients with low‐back pain, concluding that fear‐avoidance has a prognostic value for poor outcome 13. However, the results in this review were valid only in subacute pain (duration from 2 weeks to 3 months) and not in people with pain of longer duration 13. The review results are to be compared with the results in our study, where 18% of the sample with RA and long‐term pain reported stable, high fear‐avoidance beliefs. Differences in attributions of pain may explain the differences, i.e., a subsample of people with RA may be strongly influenced by the awareness of inflammatory processes in their joints and tend to manage by avoiding physical activity.
Low physical activity levels, i.e., not performing current HEPA, were a predictor identified in the present study, representing a behavioral component in the fear‐avoidance model. Furthermore, this finding is in line with studies on patients with low‐back pain 47, while it is contradicted by the results in previous cross‐sectional studies on people with RA 24, 25, possibly due to differences in samples and methods.
Anxiety and depression predicted high fear‐avoidance beliefs in the present study and are also included as key constructs in the theoretical fear‐avoidance model. This finding is not surprising, as anxiety and depression are bidirectionally correlated with pain 48, a cardinal symptom in RA. Pain intensity was not a strong predictor in the present study but still contributed significantly, in line with previous studies on people with RA 23, 24. Research on low‐back pain has explored the link between perceived pain and physical injury, suggesting patients’ uncertainty regarding diagnosis and the cause of pain explains more of the pain experience than the injury itself 28. Again, the differences in findings may be related to inflammation as a likely cause of pain in RA, whereas the physiologic triggers may not be as obvious in people with nonspecific long‐term pain.
A surprising finding, although previously reported in a cross‐sectional study of the present sample 23, was that male sex was a strong predictor of high fear‐avoidance beliefs. The reason for this sex difference is not clear, since women with RA are generally more affected by their disease and have more pain 49. Possibly traditional gender roles, with women being mainly responsible for daily household chores, necessitate early confrontation of pain and subsequent reduction of fear‐avoidance. However, this assumption has to be investigated further. Low income and education below university level predicted fear‐avoidance in the present study, which is supported by other studies identifying socioeconomic status as a consistent correlate of poor health 50. One possible explanation for these findings is that poor health literacy, with subsequent limited access to more complex information about disease and self‐management, is more frequent in groups with lower socioeconomic status 51. People with low education and low income may also more frequently have physically demanding jobs and a more sedentary leisure time 52, thus missing opportunities for nonsystematic exposure to physical activity in situations perceived as joyful.
There are methodologic limitations in this study that should be acknowledged when interpreting the results. It would have been interesting to include a sample representing all ages, severities of activity limitation, and physical activity levels. However, since data for the study were collected as part of recruitment for a physical activity intervention, our sample is not representative of the Swedish RA population most severely affected by their disease, but our proportion of women is representative, as is the age distribution 53. To further explore the links between fear‐avoidance, exercise, pain, and disability in an RA context, a more theory‐driven design would be desirable. Such a design should use data on inflammatory activity along with all variables included in the fear‐avoidance model, assess changes over time in all variables, and allow for analysis of mediators of change, e.g., in disability.
Missing data may have affected the results, but a robust method for imputation was used 54, and analyses performed both with and without imputation yielded similar results, indicating that the missingness did not bias our results. The present study cannot claim to fully explain the development of long‐term fear‐avoidance beliefs on physical activity. We recognize that there may be additional potential predictors of interest, such as catastrophizing thoughts and disease activity. Still, the study included a substantial number of variables according to a biopsychosocial approach and identified predictors of clinical relevance.
In conclusion, our results suggest that a small but significant group of people with RA hold stable, high fear‐avoidance beliefs about physical activity and may be at risk for entering into a deleterious circle of catastrophic thinking, avoidance of activities, and subsequent disuse, depression, and disability. High activity limitation, anxiety/depression, not being sufficiently physically active, and belonging to vulnerable socioeconomic groups predict high fear‐avoidance beliefs and should be considered by health professionals in their promotion of physical activity. Special attention should be given to men, as they are more likely to hold fear‐avoidance beliefs. Health professionals should assess not only medical aspects of RA but also psychological variables such as fear‐avoidance. Screening of negative pain‐related cognitions has long been included in clinical guidelines for the management of nonspecific back pain 55 and may also be useful within rheumatology. Future studies are needed to investigate whether interventions, such as graded exposure and person‐centered progressive exercise, that are effective to reduce fear‐avoidance beliefs in other chronic conditions, are also feasible and beneficial to patients with RA.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Demmelmaier had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Demmelmaier, Nordgren, Opava.
Acquisition of data
Nordgren, Opava.
Analysis and interpretation of data
Demmelmaier, Björk, Dufour, Opava.
Acknowledgments
The authors thank all participants, the Swedish Rheumatology Quality registers, and the rheumatology clinics at Danderyd Hospital (Stockholm), Karolinska University Hospital (Solna and Huddinge), Linköping University Hospital (Linköping and Norrköping), Mälarsjukhuset (Eskilstuna), Östersund Hospital (Östersund), and Sunderby Hospital (Luleå) for providing data for the study.
Supported by the Swedish Research Council, Combine Sweden, the Swedish Rheumatism Association, the National Postgraduate School of Health Care Sciences at Karolinska Institutet, and the Strategic Research Program in Health Care Sciences.
References
- 1. Scott DL, Smith C, Kingsley G. What are the consequences of early rheumatoid arthritis for the individual? Best Pract Res Clin Rheumatol 2005;19:117–36. [DOI] [PubMed] [Google Scholar]
- 2. Sokka T, Krishnan E, Häkkinen A, Hannonen P. Functional disability in rheumatoid arthritis patients compared with a community population in Finland. Arthritis Rheum 2003;48:59–63. [DOI] [PubMed] [Google Scholar]
- 3. Van Vollenhoven RF. Treatment of rheumatoid arthritis: state of the art 2009. Nat Rev Rheumatol 2009;5:531–41. [DOI] [PubMed] [Google Scholar]
- 4. Myasoedova E, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: a step forward. Curr Opin Rheumatol 2010;22:342–7. [DOI] [PubMed] [Google Scholar]
- 5. Vliet Vlieland TP, van den Ende CH. Nonpharmacological treatment of rheumatoid arthritis. Curr Opin Rheumatol 2011;23:259–64. [DOI] [PubMed] [Google Scholar]
- 6. Haskell W, Lee I, Pate R, Powell K, Blair S, Franklin B, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Assocation. Med Sci Sports Exerc 2007;39:1423–34. [DOI] [PubMed] [Google Scholar]
- 7. Sokka T, Hakkinen A, Kautiainen H, Maillefert JF, Toloza S, Hansen TM. Physical inactivity in patients with rheumatoid arthritis: data from twenty‐one countries in a cross‐sectional, international study. Arthritis Rheum 2008;59:42–50. [DOI] [PubMed] [Google Scholar]
- 8. Demmelmaier I, Bergman P, Nordgren B, Jensen I, Opava CH. Current and maintained health‐enhancing physical activity in rheumatoid arthritis: a cross‐sectional study. Arthritis Care Res (Hoboken) 2013;65:1166–76. [DOI] [PubMed] [Google Scholar]
- 9. Bauman AE, Reis RS, Sallis JF, Wells JC, Loos RJF, Martin BW. Correlates of physical activity: why are some people physically active and others not? Lancet 2012;380:258–71. [DOI] [PubMed] [Google Scholar]
- 10. Larkin L, Kennedy N. Correlates of physical activity in adults with rheumatoid arthritis: a systematic review. J Phys Act Health 2014;11:1248–61. [DOI] [PubMed] [Google Scholar]
- 11. Huffman K, Pieper C, Hall K, St Clair E, Kraus W. Self‐efficacy for exercise, more than disease‐related factors, is associated with objectively assessed exercise time and sedentary behaviour in rheumatoid arthritis. Scand J Rheumatol 2014;44:106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bandura A. Self‐efficacy. The exercise of control. New York: W. H. Freeman; 1997. [Google Scholar]
- 13. Wertli MM, Rasmussen‐Barr E, Weiser S, Bachmann LM, Brunner F. The role of fear avoidance beliefs as a prognostic factor for outcome in patients with nonspecific low back pain: a systematic review. Spine J 2014;14:816–36. [DOI] [PubMed] [Google Scholar]
- 14. Wertli MM, Rasmussen‐Barr E, Held U, Weiser S, Bachmann LM, Brunner F. Fear‐avoidance beliefs: a moderator of treatment efficacy in patients with low back pain. A systematic review. Spine J 2014;14:2658–78. [DOI] [PubMed] [Google Scholar]
- 15. Lethem J, Slade PD, Troup JD, Bentley G. Outline of a fear‐avoidance model of exaggerated pain perception: I. Behav Res Ther 1983;21:401–8. [DOI] [PubMed] [Google Scholar]
- 16. Vlaeyen JW, Linton SJ. Fear‐avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain 2000;85:317–32. [DOI] [PubMed] [Google Scholar]
- 17. Vlaeyen JW, de Jong JR, Onghena P, Kerckhoffs‐Hanssen M, Kole‐Snijders AM. Can pain‐related fear be reduced? The application of cognitive‐behavioural exposure in vivo. Pain Res Manag 2002;7:144–53. [DOI] [PubMed] [Google Scholar]
- 18. De Jong JR, Vlaeyen JW, Onghena P, Goossens ME, Geilen M, Mulder H. Fear of movement/(re)injury in chronic low back pain: education or exposure in vivo as mediator to fear reduction? Clin J Pain 2005;21:9–17. [DOI] [PubMed] [Google Scholar]
- 19. Guck TP, Burke RV, Rainville C, Hill‐Taylor D, Wallace DP. A brief primary care intervention to reduce fear of movement in chronic low back pain patients. Transl Behav Med 2015;5:113–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. De Jong JR, Vlaeyen JW, Onghena P, Cuypers C, den Hollander M, Ruijgrok J. Reduction of pain‐related fear in complex regional pain syndrome type I: the application of graded exposure in vivo. Pain 2005;116:264–75. [DOI] [PubMed] [Google Scholar]
- 21. Palstam A, Larsson A, Löfgren M, Ernberg M, Bjersing J, Bileviciute‐Ljungar I, et al. Decrease of fear avoidance beliefs following person‐centered progressive resistance exercise contributes to reduced pain disability in women with fibromyalgia: secondary exploratory analyses from a randomized controlled trial. Arthritis Res Ther 2016;18:116–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Newcomer KL, Shelerud RA, Vickers Douglas KS, Larson DR, Crawford BJ. Anxiety levels, fear‐avoidance beliefs, and disability levels at baseline and at 1 year among subjects with acute and chronic low back pain. Phys Med Rehabil 2010;2:514–20. [DOI] [PubMed] [Google Scholar]
- 23. Lööf H, Demmelmaier I, Welin Henriksson E, Lindblad S, Nordgren B, Opava CH, et al. Fear‐avoidance beliefs about physical activity in adults with rheumatoid arthritis. Scand J Rheumatol 2015;44:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundgren S, Olausson SA, Bergström G, Stenström CH. Physical activity and pain among patients with rheumatoid arthritis: a cognitive approach. Adv Physiother 2005;7:77–83. [Google Scholar]
- 25. Demmelmaier I, Dufour AB, Nordgren B, Opava CH. Trajectories of physical activity over two years in persons with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1069–77. [DOI] [PubMed] [Google Scholar]
- 26. Genolini C, Falissard B. KmL: a package to cluster longitudinal data. Comput Methods Programs Biomed 2011;104:e112–21. [DOI] [PubMed] [Google Scholar]
- 27. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 28. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear‐avoidance beliefs questionnaire (FABQ) and the role of fear‐avoidance beliefs in chronic back pain and disability. Pain 1993;52:157–68. [DOI] [PubMed] [Google Scholar]
- 29. Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain‐related fear is more disabling than pain itself: evidence on the role of pain‐related fear in chronic back pain disability. Pain 1999;80:329–39. [DOI] [PubMed] [Google Scholar]
- 30. Staal JB, Hlobil H, Koke AJ, Twisk JW, Smid T, van Mechelen W. Graded activity for workers with low back pain: who benefits most and how does it work? Arthritis Rheum 2008;59:642–9. [DOI] [PubMed] [Google Scholar]
- 31. Beneciuk J, Robinson M, George S. Low back pain subgroups using fear‐avoidance model measures: results of a cluster analysis. Clin J Pain 2012;28:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swinkels‐Meewisse I, Swinkels R, Verbeek A, Vlaeyen J, Oostendorp R. Psychometric properties of the Tampa scale for kinesiophobia and the fear‐avoidance beliefs questionnaire in acute low back pain. Man Ther 2003;8:29–36. [DOI] [PubMed] [Google Scholar]
- 33. Collins S, Moore R, McQuay H. The visual analogue pain intensity scale: what is moderate pain in millimeters? Pain 1997;72:95–7. [DOI] [PubMed] [Google Scholar]
- 34. Wolfe F, Cathey MA. The assessment and prediction of functional disability in rheumatoid arthritis. J Rheumatol 1991;18:1298–306. [PubMed] [Google Scholar]
- 35. EuroQol Group . EuroQol: a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 36. Hurst NP, Kind P, Ruta D, Hunter M, Stubbings A. Measuring health‐related quality of life in rheumatoid arthritis: validity, responsiveness and reliability of EuroQol (EQ‐5D). Br J Rheumatol 1997;36:551–9. [DOI] [PubMed] [Google Scholar]
- 37. Dzewaltowski D. Toward a model of exercise motivation. J Sport Exerc Psychol 1989;11:251–69. [Google Scholar]
- 38. Craig C, Marshall A, Sjöström M, Bauman A, Booth M, Ainsworth B. International Physical Activity Questionnaire: 12‐country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 39. Nigg C, Riebe D. The transtheoretical model: research review of exercise behavior in older adults In: Burbank P, Riebe D, editors. Promoting exercise and behavior change in older adults: interventions with the transtheoretical model. New York: Springer; 2002. p. 147–80. [Google Scholar]
- 40. Prochaska JO, DiClemente C, Norcross JC. In search of how people change. Am Psychol 1992;47:1002–4. [DOI] [PubMed] [Google Scholar]
- 41. Courneya K. Understanding readiness for regular physical activity in older individuals: an application of the theory of planned behavior. Health Psychol 1995;14:80–7. [DOI] [PubMed] [Google Scholar]
- 42. Astroth K, Fish A, Mitchell G, Bachman J, Hsueh K. Construct validity of four exercise stage of change measures in adults. Res Nurs Health 2010;33:254–64. [DOI] [PubMed] [Google Scholar]
- 43. R Core Team . R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2012. [Google Scholar]
- 44. Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non‐specific low back pain. Lancet 2012;379:482–91. [DOI] [PubMed] [Google Scholar]
- 45. Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther 2011;91:700–11. [DOI] [PubMed] [Google Scholar]
- 46. Westman A, Boersma K, Leppert J, Linton SJ. Fear‐avoidance beliefs, catastrophizing, and distress: a longitudinal subgroup analysis on patients with musculoskeletal pain. Clin J Pain 2011;27:567–77. [DOI] [PubMed] [Google Scholar]
- 47. Klenerman L, Slade P, Stanley I, Pennie B, Reilly J, Atchison L, et al. The prediction of chronicity in patients with an acute attack of low back pain in a general practice setting. Spine 1995;20:478–84. [DOI] [PubMed] [Google Scholar]
- 48. Da Silva M, Singh‐Manoux A, Brunner E, Kaffashian S, Shipley M, Kivimäki M, et al. Bidirectional association between physical activity and symptoms of anxiety and depression: the Whitehall II study. Eur J Epidemiol 2012;27:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST‐RA study. Arthritis Res Ther 2009;11:R7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hosseinpoor AR, Bergen N, Mendis S, Harper S, Verdes E, Kunst A, et al. Socioeconomic inequality in the prevalence of noncommunicable diseases in low‐ and middle‐income countries: results from the world health survey. BMC Public Health 2012;12:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sørensen K, Pelikan JM, Röthlin F, Ganahl K, Slonska Z, Doyle G, et al. Health literacy in Europe: comparative results of the European health literacy survey (HLS‐EU). Eur J Public Health 2015;25:1053–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Donoghue G, Perchoux C, Mensah K, Lakerveld J, van der Ploeg H, Bernaards C, et al. A systematic review of correlates of sedentary behaviour in adults aged 18–65 years: a socio‐ecological approach. BMC Public Health 2016;16:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neovius M, Simard JF, Askling J. Nationwide prevalence of rheumatoid arthritis and penetration of disease‐modifying drugs in Sweden. Ann Rheum Dis 2011;70:624–9. [DOI] [PubMed] [Google Scholar]
- 54. Vittinghoff E, Shiboski S, Glidden D, McCulloch C. Regression models in biostatistics: linear, logistic, survival, and repeated measures model. New York: Springer; 2005. [Google Scholar]
- 55. Kendall NA, Linton SJ, Main CJ. Guide to assessing psychosocial yellow flags in acute low back pain: risk factors for long‐term disability and work loss. Wellington: Accident Rehabilitation and Compensation Insurance Corporation of New Zealand and the National Health Committee; 1997. [Google Scholar]


