Abstract
Generalized pustular psoriasis (GPP) and erythrodermic psoriasis (EP) are the rare and severe subtypes of psoriasis, which are often difficult to treat. The aim of this phase 3, open‐label study was to evaluate efficacy and safety of guselkumab, a human interleukin‐23 monoclonal antibody, in Japanese patients with GPP and EP. Guselkumab 50 mg was administrated to GPP (n = 10) and EP (n = 11) patients at weeks 0, 4 and thereafter every 8 weeks (q8w). Beginning at week 20, patients were escalated to 100 mg q8w if they met the dose escalation criteria. The primary end‐point was the proportion of patients achieving treatment success (Clinical Global Impression score of “very much improved”, “much improved” or “minimally improved”) at week 16. Safety evaluations included assessment of treatment‐emergent adverse events (TEAE) through week 52. At week 16, the proportions of GPP and EP patients achieving treatment success were 77.8% (7/9) and 90.9% (10/11), respectively. Furthermore, guselkumab treatment consistently showed improvement in responses of secondary end‐points such as Psoriasis Area and Severity Index, Investigator's Global Assessment, Japanese Dermatological Association severity index and improvement in body surface area involvement. Improvements in quality of life, as assessed by the Dermatology Life Quality Index, were also observed through week 52. The most commonly reported TEAE was nasopharyngitis (28.6%, 6/21). Safety findings were consistent with those observed previously in other studies. In conclusion, guselkumab treatment demonstrated efficacy and showed no safety concerns in Japanese patients with GPP and EP through week 52.
Keywords: erythrodermic psoriasis, generalized pustular psoriasis, guselkumab, Japanese, long term
Introduction
Generalized pustular psoriasis (GPP) and erythrodermic psoriasis (EP) are severe and potentially life‐threatening subtypes of psoriasis, a chronic immune‐mediated inflammatory skin disease.1, 2, 3 Patients with GPP are characterized by sterile pustules on red, painful and inflamed skin4 along with pyrexia; while patients with EP typically manifest generalized erythema and scales affecting virtually the entire body.4 The overall prevalence of psoriasis in Japan was estimated to be approximately 0.3% of the general population (~430 000 in total) and nearly 1.1% and 0.4% of these have GPP and EP, respectively.5
According to Japanese guidelines, current treatments for GPP include systemic corticosteroids, cyclosporin, methotrexate, oral retinoids5, 6, 7 and biologics that target tumor necrosis factor (TNF)‐α.8 Anti‐interleukin (IL)‐17 therapies such as ixekizumab9 and brodalumab10 are also approved for GPP and EP in Japan, while secukinumab11 is approved only for GPP. Despite the availability of these treatment options, patients with GPP and EP often experience an inadequate response or poor tolerability to these drugs; hence, there is substantial need for the development of therapeutic options with novel mechanisms of action for these forms of severe and treatment‐refractory psoriasis.
Guselkumab is a human monoclonal antibody that specifically inhibits intracellular and downstream signaling of IL‐23 by binding to its p19 subunit.12 IL‐23 plays a central role in T‐helper (Th)17 cell stabilization and survival as well as production of IL‐17A, a pro‐inflammatory cytokine involved in the pathogenesis of psoriasis.12, 13 In patients with GPP, elevated levels of IL‐17A in serum and Th17‐producing cells in psoriatic skin lesions are reported.14, 15 In addition, patients with EP typically exhibit accumulation of IL‐17‐producing cells in psoriatic skin lesions similar to plaque psoriasis.16 Selective IL‐23 blockade by guselkumab may result in inhibiting pathogenic Th17 cells and consequently downstream production of multiple effector cytokines such as IL‐17A, IL‐22 and TNF‐α, and also enhance accumulation of regulatory T cells.17, 18
The present study evaluates the efficacy and safety of guselkumab in Japanese patients with GPP or EP.
Methods
Patients
Patients (≥20 years of age) diagnosed with GPP (Japan Dermatology Association [JDA] severity index, <14) or with EP having lesions covering more than 80% of the body surface area (BSA) were enrolled. Eligible patients were candidates for phototherapy or systemic treatment for psoriasis (either naive or history of such previous treatments), and EP patients were required to have a history of plaque type psoriasis. Patients were excluded if they had guttate psoriasis, drug‐induced psoriasis, a history of malignancy (except for non‐melanoma skin cancer) within 5 years of screening, or a history or current signs of any severe, progressive or uncontrolled medical conditions. Patients who had received any of the following treatments prior to first administration of study agent were also excluded: adalimumab within 8 weeks; infliximab within 12 weeks; agents targeting IL‐12, IL‐17 or IL‐23 within 6 months; granulocyte and monocyte apheresis therapy within 3 months; or systemic immunosuppressants or phototherapy within 4 weeks.
An independent data monitoring committee with access to unblinded data monitored the safety of the study participants until the week‐52 database lock. The study protocol was approved by the local institutional review board and the study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization and Good Clinical Practise guidelines. All patients provided written informed consent before participating in this study. This study was registered at clinicaltrials.gov (NCT02343744).
Study design
This phase 3, single‐arm, open‐label, multicenter study was conducted at 23 sites in Japan from 28 January 2015 to 12 August 2016. The study consisted of a 6‐week screening phase, a 52‐week open‐label treatment phase and a long‐term extension phase. Guselkumab 50 mg was administrated s.c. at weeks 0, 4 and every 8 weeks (q8w) thereafter until week 52 (Fig. 1a). Dose escalation was permitted for patients who were assessed as having a Clinical Global Impression (CGI)19 rating of “no change” or “worsened” at any scheduled study visit beginning at week 20, as well as for patients assessed as CGI “minimally improved” depending on investigator discretion. Patients undergoing dose escalation received guselkumab 100 mg and continued to receive the 100 mg q8w dose regimen until the study end. Concomitant topical therapies were permitted throughout the study and use of methotrexate or retinoids was also permitted if initiated 1 week before the first administration of study agent, preferably at unchanged dose throughout the study. Agents targeting TNF‐α, IL‐12/23 or IL‐23, IL‐17, α4‐integrin antagonists or any other biologic agents, systemic steroids and other conventional systemic therapies (except methotrexate or retinoids) were prohibited.
Figure 1.
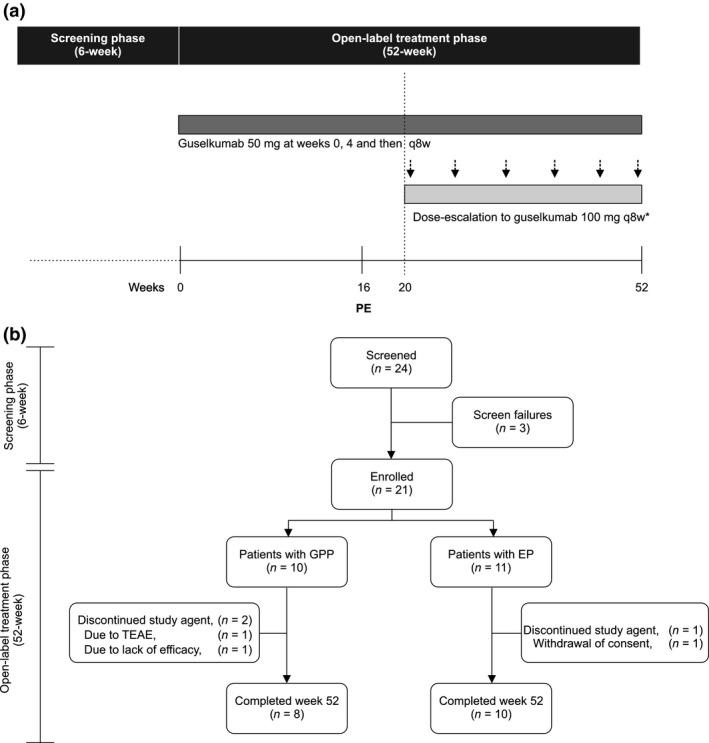
(a) Study design. *After week 20, patients who were defined “no change” or “worsened” in Clinical Global Impression received guselkumab 100 mg and patients who were defined as “minimally improved” in Clinical Global Impression received a 100 mg dose based on the investigator's decision. (b) Patient disposition. PE, primary end‐point; TEAE, treatment‐emergent adverse event.
Efficacy end‐points
The primary efficacy end‐point was the proportion of patients achieving treatment success, defined as a CGI score of “very much improved”, “much improved” or “minimally improved” at week 16. The CGI score is defined as 1 = “very much improved”, 2 = “much improved”, 3 = “minimally improved”, 4 = “no change”, 5 = “minimally worse”, 6 = “much worse” and 7 = “very much worse” since the initiation of treatment (Table S1).20 In the present study, CGI scores of “minimally worse”, “much worse” and “very much worse” are combined into “worsened”.
The secondary efficacy end‐points were assessed through week 52 and were as follows: proportion of patients achieving treatment success over time; change from baseline in JDA severity index total score (0 [best] to 17 [worst]) and component score (skin symptoms and systemic symptoms/laboratory findings) for patients with GPP; change from baseline in BSA involvement for patients with EP; the proportion of patients achieving an Investigator's Global Assessment (IGA) score of cleared or minimal (0/1); percentage improvement in Psoriasis Area and Severity Index (PASI); change from baseline in the Dermatology Life Quality Index (DLQI)21 and percentage of patients achieving DLQI 0/1 among those with a baseline DLQI score of more than 1; percentage of patients with a reduction of 5 points or more in the DLQI; and change from baseline in the Physical Component Scores (PCS), Mental Component Scores (MCS) of the 36‐item Short‐Form Health Assessment Questionnaire (SF‐36).22
Safety assessments
Safety assessments included treatment‐emergent adverse events (TEAE), including injection site and allergic reactions, serious TEAE, electrocardiograms, vital signs, physical examinations, concomitant medications reviews and tuberculosis testing. Determination of the toxicity grade of clinical laboratory parameters was based on Common Terminology Criteria for Adverse Events (CTCAE) grading. The presence of antibodies to guselkumab in serum was evaluated using a validated electrochemiluminescence immunoassay method using the Meso Scale Discovery (MSD) platform.
Statistical analysis
Considering the low prevalence of GPP and EP among Japanese patients (1.1% and 0.4%, respectively, of the entire psoriatic population in Japan), a sample size of approximately 20 patients (10 patients each for GPP and EP) was considered feasible for the study. All patients who received at least one dose of guselkumab by s.c. injection were included in both the efficacy analysis set and the safety analysis set. Data for continuous variables were summarized by descriptive statistics, and categorical data were summarized by absolute counts and percentages. No statistical tests were performed. Missing data were treated as such and imputation for missing data was not employed. This report includes data collected up to week 52.
Results
Of the 24 patients who were screened, 21 patients (GPP, n = 10 and EP, n = 11) were enrolled and received the study agent. Of these 21 patients, 18 completed the week‐52 visit (GPP, n = 8 and EP, n = 10). Two patients with GPP discontinued study treatment, one due to a serious adverse event of squamous cell carcinoma of the skin and the other due to lack of efficacy (underwent dose escalation at week 20 but discontinued following 28 weeks of treatment) and one patient with EP withdrew consent to participate (Fig. 1b).
Study participants were mostly men (16/21, 76.2%) and had a mean age of 48.9 years (standard deviation [SD] = 14.63) and body mass index of 24.8 kg/m2 (SD = 5.40) (Table 1). Median disease duration was 9 years (range, 0–35 years) (Table 1). Patients with GPP had JDA severity index scores of either “mild” (0–6; n = 8) or “moderate” (7–10, n = 2) at baseline. Among patients with EP (n = 11), median baseline BSA was 85% (range, 80–97%) (Table 1).
Table 1.
Patient demographics and baseline characteristics
| Characteristic | GPP (n = 10) | EP (n = 11) | GPP + EP (n = 21) |
|---|---|---|---|
| Age, mean (SD), (years) | 42.6 (8.97) | 54.6 (16.72) | 48.9 (14.63) |
| Sex, male, n (%) | 6 (60.0) | 10 (90.9) | 16 (76.2) |
| BMI, mean (SD), (kg/m2), | 26.9 (6.39) | 23.0 (3.69) | 24.8 (5.40) |
| Disease duration, median (range), years | 14.9 (0; 31) | 5.0 (1; 35) | 9.0 (0; 35) |
| JDA severity index (0–17), n (%) | |||
| Mild (0–6) | 8 (80.0) | – | – |
| Moderate (7–10) | 2 (20.0) | – | – |
| Severe (11–17) | 0 | – | – |
| Mean (SD) | 5.4 (1.78) | – | – |
| Involvement of body surface area, mean (SD), % | – | 86.0 (5.39) | – |
| PASI total score (0–72), mean (SD) | 29.3 (19.95) | 40.9 (10.24) | 35.4 (16.34) |
| DLQI (0–30), mean (SD) | 10.1 (6.24) | 9.8 (6.85) | 10.0 (6.41) |
| SF‐36 PCS, mean (SD) | 38.6 (18.47) | 49.6 (10.43) | 44.4 (15.50) |
| SF‐36 MCS, mean (SD) | 40.6 (12.66) | 47.9 (11.49) | 44.4 (12.33) |
| IGA total average score, n (%) | |||
| Minimal (1) | 0 | 0 | 0 |
| Mild (2) | 5 (50.0) | 3 (27.3) | 8 (38.1) |
| Moderate (3) | 3 (30.0) | 6 (54.5) | 9 (42.9) |
| Severe (4) | 2 (20.0) | 2 (18.2) | 4 (19.0) |
| Prior systemic therapies, n (%) | 7 (70) | 6 (54.5) | 13 (61.9) |
| Topical agent | 10 (100) | 11 (100) | 21 (100) |
| Phototherapy, n (%) | 7 (70) | 6 (54.5) | 13 (61.9) |
| UV‐B treatment | 5 (50) | 5 (45.5) | 10 (47.6) |
| PUVA treatment | 3 (30) | 2 (18.2) | 5 (23.8) |
| Systemic therapies, n (%) | |||
| Cyclosporin | 7 (70) | 6 (54.5) | 13 (61.9) |
| Methotrexate | 4 (40) | 4 (36.4) | 8 (38.1) |
| Biologics, n (%) | 3 (30) | 3 (27.3) | 6 (28.6) |
| Infliximab | 3 (30) | 1 (9.1) | 4 (19) |
| Ixekizumab | 0 | 1 (9.1) | 1 (4.8) |
| Adalimumab | 1 (10) | 0 | 1 (4.8) |
| Secukinumab | 0 | 1 (9.1) | 1 (4.8) |
| Concomitant medication, N | |||
| Non‐corticosteroids | 10 | 10 | 20 |
| Topical corticosteroids | 7 | 8 | 15 |
| Systemic immunosuppressive therapy | 4 | 2 | 6 |
BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis; IGA, Investigator's Global Assessment; JDA, Japanese Dermatological Association; MCS, Mental Component Summary; PASI, Psoriasis Area and Severity Index; PCS, Physical Component Summary; PUVA, psoralen plus ultraviolet A therapy; SD, standard deviation; SF‐36, 36‐Item Short Form Health Assessment Questionnaire; UV‐B, narrowband ultraviolet B therapy.
Prior treatment included topical treatment (n = 21, 100%), phototherapy (n = 13, 61.9%), systemic therapies such as cyclosporin (n = 13, 61.9%) and methotrexate (n = 8, 38.1%), and biologics such as anti‐TNF‐α agents (infliximab, n = 4 [19%] and adalimumab, n = 1 [4.8%]) and IL‐17 inhibitors (ixekizumab and secukinumab, n = 1 [4.8%] each).
Primary efficacy outcomes
By week 16, the majority of GPP and EP patients showed signs of improvement. A total of seven out of nine evaluable GPP patients achieved treatment success (CGI “very much improved” [n = 2, 22.2%] or “much improved” [n = 2, 22.2%] or “minimally improved” [n = 3, 33.3%]) at week 16. Similarly, 10 out of 11 evaluable EP patients achieved treatment success (“very much improved” [n = 5, 45.5%], “much improved” [n = 3, 27.3%] or “minimally improved” [n = 2, 18.2%]) (Fig. 2). The treatment success rates were 77.8% and 90.9% in GPP and EP, respectively. As noted in the photograph (Fig. 3) of a GPP patient and EP patient with CGI of “much improved”, near complete clearance of skin lesion was observed at week 28 and week 52.
Figure 2.
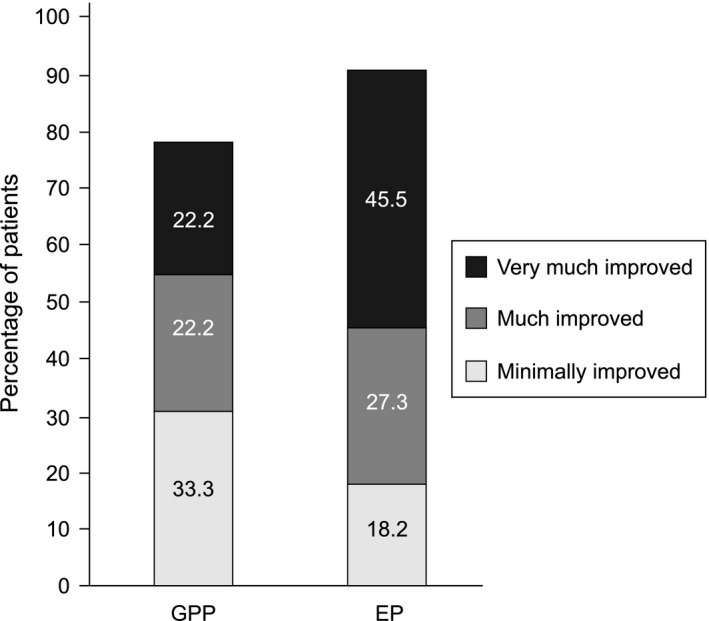
Treatment success assessment for GPP and EP patients at week 16 (CGI: efficacy analysis set). Treatment success is defined as “very much improved”, “much improved” or “minimally improved” in CGI. Percentages calculated with the number of assessed patients in each group as denominator. CGI, Clinical Global Impression; EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis.
Figure 3.
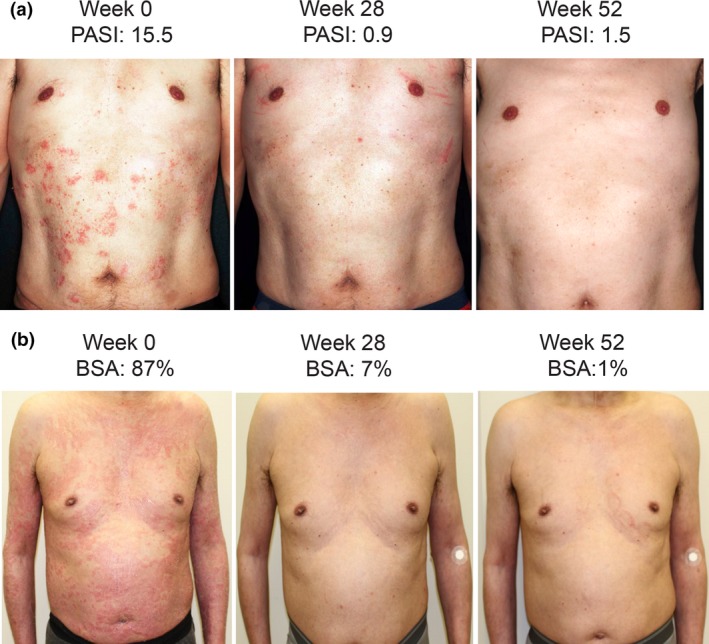
Photographs of patients with (a) GPP and (b) EP taken at baseline (week 0), 28 weeks and 52 weeks of treatment with guselkumab. BSA, body surface area; CGI, Clinical Global Impression; EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis; PASI, Psoriasis Area and Severity Index.
Secondary efficacy outcomes
GPP patients
Treatment success was observed as early as week 1 in patients with GPP (5/10, 50%) and response was maintained through week 52 in patients who did not undergo dose escalation as well as those who had dose escalation. Treatment success was 100% (8/8) for all patients who completed the study at week 52 (Fig. 4).
Figure 4.
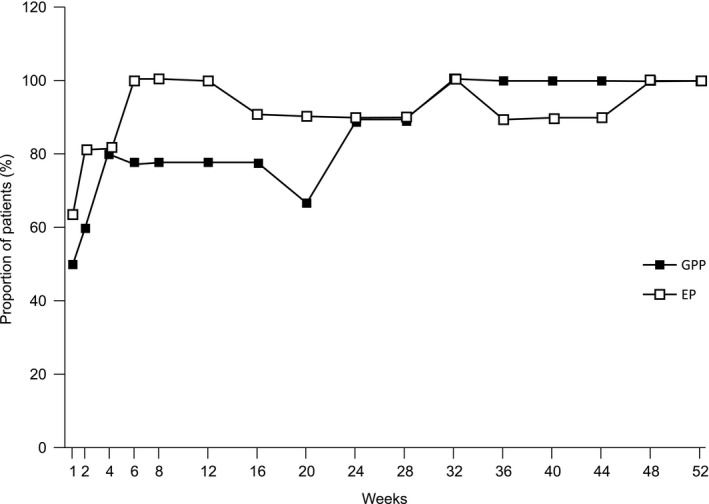
Treatment success over time through week 52 (CGI: efficacy analysis set). Treatment success is defined as “very much improved”, “much improved” or “minimally improved” in CGI. CGI, Clinical Global Impression; EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis.
At week 20, five patients with GPP who had a CGI response of “minimally improved” (n = 2), “no change” (n = 1) or “worsened” (n = 2) underwent dose escalation. Among these patients, the treatment success rate increased from 40.0% (n = 2/5) at week 20% to 100% (n = 4/4) at week 52.
Reduction in the mean baseline Psoriasis Area and Severity Index (PASI) was also observed at week eight among GPP patients (n = 9; ∆, −13.8 [SD = 12.68]). At week 52, GPP patients (n = 8) achieved a mean absolute PASI of 4.8 (SD = 6.41) with median percent improvement of 86.8%. Improvement in PASI total score in each GPP patient (n = 10) is presented in Figure 5(a). Treatment response based on IGA score of 0/1 was noted at week 8 in GPP patients (7/9, 77.8%) and the response was maintained through week 52 (Table 2).
Figure 5.

(a) PASI total score over time in GPP patients through week 52 (n = 10). (b) JDA severity index total score over time in GPP patients through week 52 (n = 10). GPP, generalized pustular psoriasis; JDA, Japanese Dermatological Association; PASI, Psoriasis Area Severity Index.
Table 2.
Secondary efficacy outcomes of guselkumab treatment in patients with GPP and EP through week 52 (efficacy analysis set)
| Change from baseline | GPP | EP | ||||
|---|---|---|---|---|---|---|
| 50 mg (n = 4) | 100 mg (n = 4) | Combined (n = 8) | 50 mg (n = 8) | 100 mg (n = 2) | Combined (n = 10) | |
| JDA, mean (SD) | −3.5 (2.38) | −2.5 (2.65) | −3.0 (2.39) | NA | NA | NA |
| BSA, mean (SD) | NA | NA | NA | −81.0 (9.67) | −45.0 (9.90) | −73.8 (17.72) |
| IGA (score 0 or 1), n (%) | 4 (100.0) | 3 (75.0) | 7 (87.5) | 7 (87.5) | 1 (50.0) | 8 (80.0) |
| PASI total score, mean (SD) | −18.9 (14.70) | −25.6 (11.85) | −22.3 (12.87) | −41.4 (10.32) | −18.9 (7.07) | −36.9 (13.36) |
| DLQI total score, mean (SD) | −9.0 (6.68) | −5.8 (4.57) | −7.4 (5.58) | −8.0 (6.93) | −5.0 (4.24) | −7.4 (6.40) |
| Reduction of ≥5 points, n (%) | 3 (75.0) | 3 (75.0) | 6 (75.0) | 5 (62.5) | 1 (50.0) | 6 (60.0) |
| SF‐36 PCS, mean (SD) (week 48) | 12.2 (17.51) | 14.8 (6.46) | 13.5 (12.30) | 1.2 (8.83) | −12.9 (9.97) | −1.6 (10.35) |
| SF‐36 MCS, mean (SD) (week 48) | 8.0 (13.82) | 6.6 (11.23) | 7.3 (11.68) | 8.3 (8.08) | 5.7 (0.40) | 7.8 (7.21) |
The baseline measurement is defined as the last measurement taken prior to or at the date of the first study agent administration at week 0. Percentages calculated with the number of assessed patients in each group per visit as denominator. BSA, body surface area; DLQI, Dermatology Life Quality Index; EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis; IGA, Investigator's Global Assessment; JDA, Japanese Dermatological Association; MCS, Mental Component Summary; PCS, Physical Component Summary; PASI, Psoriasis Area and Severity Index; SD, standard deviation; SF‐36, 36‐Item Short‐Form Health Assessment Questionnaire.
Reduction in JDA severity index total score from baseline was observed as early as week 1 (mean, −0.2 [SD = 2.04]) and continued to decrease throughout the study period (week 20, −2.0 [SD = 2.60]; week 36, −3.3 [SD = 1.91]; and week 52, −3.0 [SD = 2.39]). At week 52, a JDA severity index total score of “mild” in seven of the eight (87.5%) patients and “moderate” in one was noted. The JDA severity index total score through week 52 in each GPP patient (n = 10) is presented in Figure 5(b).
EP patients
After initiating guselkumab treatment, seven of the 11 (63.6%) EP patients achieved treatment success as early as week 1. Response was achieved by 100% (n = 10/10) of EP patients at week 52 for both dose escalated and non‐dose‐escalated patients. Two EP patients underwent dose escalation; treatment success was achieved by one at week 20 and by both patients at week 52.
At week 8, the reduction in the mean baseline PASI was observed in EP patients (n = 11; ∆, −26.3 [SD = 11.64]). At week 52, EP patients (n = 10) achieved a mean absolute PASI of 3.9 (SD = 4.27) with median percent improvement of 94.1%. Improvement in PASI total score in each EP patient (n = 11) is presented in Figure 6(a).
Figure 6.
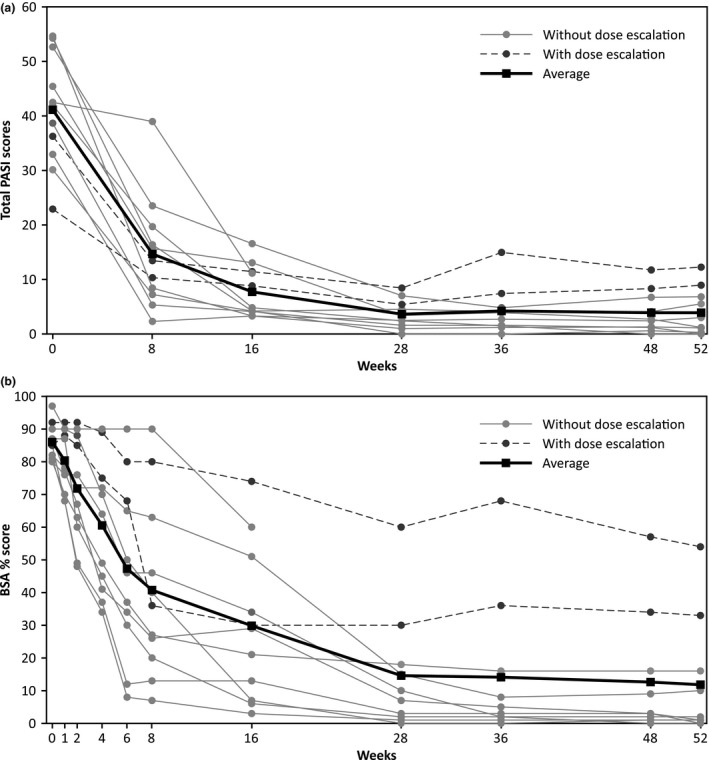
(a) PASI total score over time in EP patients through week 52 (n = 11). (b) BSA involvement by psoriatic lesions over time in EP patients through week 52 (n = 11). BSA, body surface area; EP, erythrodermic psoriasis; PASI, Psoriasis Area Severity Index.
The treatment response based on IGA 0/1 was noted at week 8 in EP patients (n = 4/11, 36.4%) and the improvement was observed through week 52, with eight out of 10 EP patients achieving IGA 0/1 (Table 2).
In patients with EP, the mean baseline BSA was reduced from 86% (SD = 5.39) to 7% (SD = 6.76), namely by 67% at week 28, and this level of reduction was maintained through week 52. The BSA of involvement of lesion in each EP patient (n = 11) through week 52 is presented in Figure 6(b).
DLQI and SF‐36
Improvement in DLQI was also observed in both GPP and EP patients as early as week 8. Mean baseline DLQI score improved from 10.1 (SD = 6.24) at baseline to 6.2 (SD = 8.04) at week 8, and eventually to 0.5 (SD = 0.58) at week 52 in GPP patients. Similarly in EP patients, mean baseline DLQI score improved from 9.8 (SD = 6.85) to 4.6 (SD = 5.50) at week 8, and eventually to 1.1 (SD = 1.73) at week 52. A DLQI score of 0/1 (indicating no impact of disease on quality of life [QoL]) at week 52 was achieved by 42.9% (3/7) of patients with GPP and 66.7% (6/9) of patients with EP. Improvement in health‐related QoL as measured by the MCS and the PCS the SF‐36 was also observed through week 48 in both patients with GPP and EP (Table 2).
Prior treatment with biologics
A total of six patients (GPP, n = 3 and EP, n = 3) had prior treatment with at least one anti‐TNF‐α agents and/or IL‐17 inhibitors. Of these patients, five (GPP, n = 3 and EP, n = 2) experienced treatment success at week 16.
Safety outcomes
All 21 patients enrolled in the study experienced at least one TEAE through week 52. After dose escalation to 100 mg q8w, three of the five patients with GPP and both patients with EP experienced at least one TEAE. The most common TEAE reported overall were nasopharyngitis (6/21, 28.6%), gastroenteritis, nausea, arthralgia and alopecia (2/21, 9.5% each) (Table 3).
Table 3.
Safety outcomes through week 52 (safety analysis set)
| Adverse event, n (%) | GPP | EP | GPP + EP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 50 mg q8w | 50 mg q8w →100 mg q8w | GPP combined | 50 mg q8w | 50 mg q8w →100 mg q8w | EP combined | 50 mg q8w | 50 mg q8w →100 mg q8w | GPP + EP | |
| n | 10 | 5 | 10 | 11 | 2 | 11 | 21 | 7 | 21 |
| Patients with ≥1 TEAE | 9 (90.0) | 3 (60.0) | 10 (100.0) | 11 (100.0) | 2 (100.0) | 11 (100.0) | 20 (95.2) | 5 (71.4) | 21 (100.0) |
| TEAE of severe intensity | 1 (10.0) | 0 | 1 (10.0) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (4.8) |
| Treatment‐emergent infections | 5 (50.0) | 1 (20.0) | 5 (50.0) | 9 (81.8) | 0 | 9 (81.8) | 14 (66.7) | 1 (14.3) | 14 (66.7) |
| TEAE by preferred term | |||||||||
| Nasopharyngitis | 2 (20.0) | 0 | 2 (20.0) | 4 (36.4) | 0 | 4 (36.4) | 6 (28.6) | 0 | 6 (28.6) |
| Gastroenteritis | 0 | 1 (20.0) | 1 (10.0) | 1 (9.1) | 0 | 1 (9.1) | 1 (4.8) | 1 (14.3) | 2 (9.5) |
| Nausea | 1 (10.0) | 0 | 1 (10.0) | 0 | 1 (50.0) | 1 (9.1) | 1 (4.8) | 1 (14.3) | 2 (9.5) |
| Arthralgia | 0 | 1 (20.0) | 1 (10.0) | 0 | 1 (50.0) | 1 (9.1) | 0 | 2 (28.6) | 2 (9.5) |
| Alopecia | 2 (20.0) | 0 | 2 (20.0) | 0 | 0 | 0 | 2 (9.5) | 0 | 2 (9.5) |
| Serious TEAE | 2 (20.0) | 0 | 2 (20.0) | 1 (9.1) | 0 | 1 (9.1) | 3 (14.3) | 0 | 3 (14.3) |
| Fall | 1 (10.0) | 0 | 1 (10.0) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (4.8) |
| Rib fracture | 0 | 0 | 0 | 1 (9.1) | 0 | 1 (9.1) | 1 (4.8) | 0 | 1 (4.8) |
| Squamous cell carcinoma | 1 (10.0) | 0 | 1 (10.0) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (4.8) |
| Loss of consciousness | 1 (10.0) | 0 | 1 (10.0) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (4.8) |
| TEAE leading to study drug discontinuation | 1 (10.0) | 0 | 1 (10.0) | 0 | 0 | 0 | 1 (4.8) | 0 | 1 (4.8) |
EP, erythrodermic psoriasis; GPP, generalized pustular psoriasis; TEAE, treatment‐emergent adverse event.
In total, four serious TEAE were reported in three of the 21 patients: two GPP patients (n = 1, fall and loss of consciousness; n = 1, squamous cell carcinoma of skin) and one EP patient (rib fracture). The GPP patient who experienced the serious TEAE of squamous cell carcinoma of the skin on day 29 discontinued the study agent on the same day. This patient had received phototherapy for psoriasis prior to study participation. All TEAE were mild to moderate in severity except for the event of squamous cell carcinoma, which was considered to be severe. The dose‐escalated patients (100 mg q8w) did not experience any serious TEAE or any TEAE leading to study discontinuation. TEAE of tinea pedis in one GPP patient and abnormal hepatic function in one EP patient were considered “reasonably related” to the study agent. No deaths or injection‐site reactions were reported. There was no new occurrence of psoriasis observed in any patient through week 52, although one GPP patient experienced worsening of psoriasis with mild severity on day 3 after the first dose administration.
There were no clinically meaningful changes or time‐related patterns observed in laboratory assessments, vital signs and electrocardiogram through week 52. For pre‐specified laboratory parameters of special interest, such as neutrophils, platelets and liver functions tests, a post‐baseline worse CTCAE grade of 2 or more was noted in three EP patients, one with grade 2 alanine aminotransferase (ALT) elevation and two with grade 3 aspartate aminotransferase (AST) elevation. These three patients had a comorbidity of chronic liver disease, such as fatty liver or alcohol‐induced liver disease. Both patients with a grade 3 AST elevation had an abnormal AST level at baseline. None of these laboratory test shifts were associated with a clinical diagnosis of hepatitis and all patients continued in the study. No patient was found to be positive for antibodies against guselkumab through week 52.
Discussion
In this 52‐week, phase 3, open‐label study, treatment with guselkumab demonstrated clinical improvement of GPP and EP in Japanese patients. The primary efficacy end‐point (treatment success at week 16 based on CGI response) was achieved by the majority of Japanese patients with GPP and EP. Guselkumab treatment exhibited rapid onset of action, with response observed as early as 1 week post‐treatment, and a consistent pattern of improvement in several secondary efficacy end‐points was seen. Efficacy outcomes, such as the JDA severity index for GPP, BSA involvement for EP, absolute PASI and IGA 0/1 response, consistently improved following treatment with guselkumab through week 52. Treatment with guselkumab also improved health‐related QoL as measured by achieving DLQI 0/1, and SF‐36 MCS and PCS responses through week 52. Overall, guselkumab treatment demonstrated an efficacy in Japanese patients with GPP and EP through week 52, consistent with observations from the global development program for guselkumab in moderate to severe plaque psoriasis.20, 23
Some patients did not have an adequate clinical response to administration of 50 mg and underwent dose escalation to 100 mg at week 20. Among these patients, the treatment success rate increased consistently from week 20 through week 52. In addition, no notable safety findings were observed in patients who underwent dose escalation to 100 mg. In view of this, a dose of 50 mg may be insufficient for some patients with GPP or EP, and generally a 100‐mg dose may be preferable for GPP and EP patients.
A total of six patients (three GPP and three EP patients) had prior treatment with biologics such as anti‐TNF‐α agents and IL‐17 inhibitors. Treatment benefit was observed in a limited number of patients who had prior treatment with anti‐TNF or anti‐IL‐17 biologics. Guselkumab with a mechanism of action distinct from that of anti‐TNF‐α agents and IL‐17 inhibitors may improve the symptoms of GPP and EP patients with prior exposure to biologics.
During the natural course of the disease, GPP patients may often experience worsening of systemic and pustular signs and symptoms. In this study, treatment with guselkumab through week 52 prevented such exacerbations, except in one patient who had worsening of psoriasis of mild severity. There were no unexpected safety signals for guselkumab in Japanese patients with GPP and EP. There were no deaths, injection‐site reactions, anti‐guselkumab antibodies or serious infections reported in the study. The overall safety results of this study in Japanese patients treated with guselkumab were consistent with the previous studies conducted in a global population with moderate to severe plaque psoriasis23 and in Japanese patients with moderate to severe psoriasis24 and palmoplantar pustulosis.25
Limitations, such as small size of population per disease condition, single‐arm open‐label study design, lack of control group and statistical comparison, should be considered when interpreting the results. Despite these limitations, the treatment benefits of the present study suggests the novel therapy option for the patients with GPP and EP.
In conclusion, data from this study demonstrate the clinical benefit of guselkumab in treating GPP and EP patients. Efficacy was achieved and maintained through week 52 and the safety profile of guselkumab was consistent with previous studies for guselkumab in psoriasis, establishing a positive benefit–risk profile for treating GPP and EP patients. These results suggest that selective blockade of IL‐23 using guselkumab may be a viable treatment option in the management of GPP and EP.
Conflict of Interest
S. S. has received honoraria as an advisory board member and/or speaker and/or speaker/faculty education from Abbvie, Mitsubishi‐Tanabe Pharma, Eisai, Novartis, Janssen, Kyowa‐Kirin and Eli Lilly Japan. H. N. has received honoraria and/or research grants as an advisory board member and/or speaker from ABC Pharma, Kyowa Hakko Kirin, Abbvie, Mitsubishi‐Tanabe Pharma, LEO Pharma, Maruho, Eli Lilly Japan and Janssen. H. K., H. M., R. G. and R. Z. are employees of Janssen Pharmaceutical, Tokyo, Japan. Author contributions were as follows: H. N. was involved in conception or design of the work; S. S. and R. Z. were involved in acquisition of the data and analysis, respectively; all authors except S. S. were involved in interpretation of data; all authors were also involved in drafting the article or revising it critically for important intellectual content, for final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Supporting information
Table S1. Clinical Global Impression scale.19
Acknowledgments
The authors thank the study participants without whom this study would never have been accomplished and also thank the investigators for their participation in the study. Writing assistance was provided by Vaishali Jadhav and additional editorial support was provided by Ramji Narayan (both SIRO Clinpharm, Thane, India). Publication support was provided by Kenichiro Tsutsumi (Janssen Pharmaceutical, Japan). This study was funded by Janssen Pharmaceutical, Japan.
Previous presentation: The week 28 results of this study were presented at the 75th American Academy of Dermatology Annual Meeting, 3–7 March 2017, Orlando, Florida, USA.
References
- 1. Kawada A, Tezuka T, Nakamizo Y et al A survey of psoriasis patients in Japan from 1982 to 2001. J Dermatol Sci 2003; 31(1): 59–64. [DOI] [PubMed] [Google Scholar]
- 2. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370(9583): 263–271. [DOI] [PubMed] [Google Scholar]
- 3. Rapp SR, Feldman SR, Exum ML, Fleischer AB, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41(3): 401–407. [DOI] [PubMed] [Google Scholar]
- 4. Weigle N, McBane S. Psoriasis. Am Fam Physician 2013; 87(9): 626–633. [PubMed] [Google Scholar]
- 5. Kubota K, Kamijima Y, Sato T et al Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open 2015; 5(1): e006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haustein UF, Rytter M. Methotrexate in psoriasis: 26 years’ experience with low‐dose long‐term treatment. J Eur Acad Dermatol Venereol 2000; 14(5): 382–388. [DOI] [PubMed] [Google Scholar]
- 7. Iwatsuki K, Terui T, Ozawa A et al Practice Guidelines 2010 for generalized pustular psoriasis (GPP): treatment guidelines incorporating TNF‐α inhibitor. Jpn J Dermatol 2010; 120: 815–839. [Google Scholar]
- 8. Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol 2016; 152(7): 825–828. [DOI] [PubMed] [Google Scholar]
- 9. Saeki H, Nakagawa H, Nakajo K et al Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52‐week, open‐label, phase 3 study (UNCOVER‐J). J Dermatol 2017; 44(4): 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yamasaki K, Nakagawa H, Kubo Y, Ootaki K. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52‐week, open‐label study. Br J Dermatol 2017; 176(3): 741–751. [DOI] [PubMed] [Google Scholar]
- 11. Imafuku S, Honma M, Okubo Y et al Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52‐week analysis from phase III open‐label multicenter Japanese study. J Dermatol 2016; 43(9): 1011–1017. [DOI] [PubMed] [Google Scholar]
- 12. Levin AA, Gottlieb AB. Specific targeting of interleukin‐23p19 as effective treatment for psoriasis. J Am Acad Dermatol 2014; 70(3): 555–561. [DOI] [PubMed] [Google Scholar]
- 13. Fitch E, Harper E, Skorcheva I, Kurtz SE, Blauvelt A. Pathophysiology of psoriasis: recent advances on IL‐23 and Th17 cytokines. Curr Rheumatol Rep 2007; 9(6): 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yasukawa S, Dainichi T, Kokuba H et al Bullous pemphigoid followed by pustular psoriasis showing Th1, Th2, Treg and Th17 immunological changes. Eur J Dermatol 2009; 19(1): 69–71. [DOI] [PubMed] [Google Scholar]
- 15. Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL‐17 in different clinical subtypes of psoriasis. Arch Dermatol Res 2012; 304(6): 465–469. [DOI] [PubMed] [Google Scholar]
- 16. Moy AP, Murali M, Kroshinsky D, Duncan LM, Nazarian RM. Immunologic overlap of helper T‐cell subtypes 17 and 22 in erythrodermic psoriasis and atopic dermatitis. JAMA Dermatol 2015; 151(7): 753–760. [DOI] [PubMed] [Google Scholar]
- 17. Di Meglio P, Nestle FO. The role of IL‐23 in the immunopathogenesis of psoriasis. F1000 Biol Rep 2010; 2: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maxwell JR, Zhang Y, Brown WA et al Differential roles for interleukin‐23 and interleukin‐17 in intestinal immunoregulation. Immunity 2015; 43(4): 739–750. [DOI] [PubMed] [Google Scholar]
- 19. Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the clinical global impressions (CGI) scale for use in bipolar illness (BP): the CGI‐BP. Psychiatry Res 1997; 73(3): 159–171. [DOI] [PubMed] [Google Scholar]
- 20. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007; 4(7): 28–37. [PMC free article] [PubMed] [Google Scholar]
- 21. Finlay AY, Khan G. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol 1994; 19(3): 210–216. [DOI] [PubMed] [Google Scholar]
- 22. Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36): I. Conceptual framework and item selection. Med Care 1992; 30(6): 473–483. [PubMed] [Google Scholar]
- 23. Gordon KB, Duffin KC, Bissonnette R et al A phase 2 trial of guselkumab versus adalimumab for plaque psoriasis. N Engl J Med 2015; 373(2): 136–144. [DOI] [PubMed] [Google Scholar]
- 24. Nemoto O, Hirose K, Shibata S, Li K, Kubo H. Safety and efficacy of Guselkumab in Japanese patients with moderate‐to‐severe plaque psoriasis: a randomized, placebo‐controlled, ascending‐dose study. Br J Dermatol 2018; 178: 689–696. [DOI] [PubMed] [Google Scholar]
- 25. Terui T, Kobayashi S, Okubo Y, Murakami M, Hirose K, Kubo H. Efficacy and safety of guselkumab, an anti‐interleukin 23 monoclonal antibody, for palmoplantar pustulosis. JAMA Dermatol 2018; https://doi.org/10.1001/jamadermatol.2017.5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Global Impression scale.19


