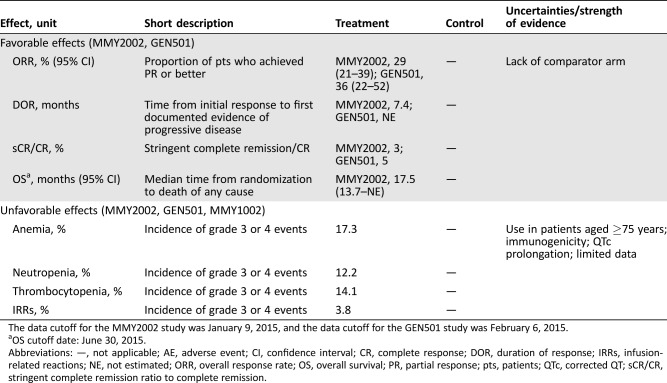
Table 1. Key favorable and unfavorable effects of daratumumab for the treatment of adult patients with relapsed and refractory multiple myeloma, whose prior therapy included a proteasome inhibitor and an immunomodulatory agent and who demonstrated disease progression on the last therapy.
The data cutoff for the MMY2002 study was January 9, 2015, and the data cutoff for the GEN501 study was February 6, 2015.
OS cutoff date: June 30, 2015.
Abbreviations: —, not applicable; AE, adverse event; CI, confidence interval; CR, complete response; DOR, duration of response; IRRs, infusion‐related reactions; NE, not estimated; ORR, overall response rate; OS, overall survival; PR, partial response; pts, patients; QTc, corrected QT; sCR/CR, stringent complete remission ratio to complete remission.